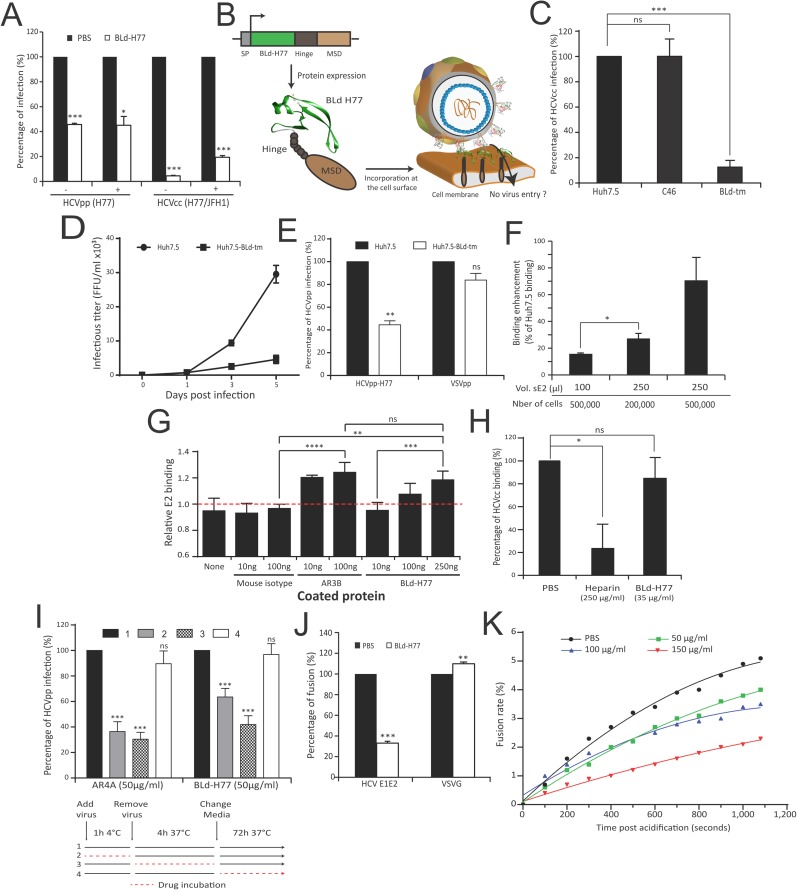
Fig 5. A transmembranous form of BLd-H77 inhibits HCV infection.
(A) BLd acts onto viral particles. H77 HCVpp or H77/JFH-1 HCVcc particles were pre-incubated with BLd-H77 (50μg/ml or 35μg/ml, respectively) or with PBS. Prior to infection of Huh7.5 cells, HCV particles and BLd-H77 mixes were diluted (1/5; +) or not (-) with cell culture media resulting in a BLd-H77 concentrations inhibiting infectivity by less than 20% (see Fig 4F and Fig 4H). Viral titers of primary infection were determined 72h (HCVpp) or 4 days (HCVcc) post-infection and expressed as percentages of infection according to PBS control experiments (mean ± SD; n = 3). Statistical significances (*p<0.05, ***p<0.001) were determined for each experimental condition versus control condition (100%). (B) Schematic representation of the BLd-H77 transmembrane form (BLd-tm) and its possible mode of action. An IgG2 hinge region (Hinge) and the transmembrane region of the CD34 molecule (MSD) were added to the C-terminal of BLd-H77. SP, signal peptide. (C) A BLd-H77 transmembranous form inhibits HCVcc infection. H77/JFH-1 HCVcc were used to infect non-transduced Huh7.5, Huh7.5-C46 (C46) and Huh7.5-BLd-tm (BLd-tm). Four days post-infection, FFU titers were determined for each cell type and percentages of primary infection were normalized according to the viral titers determined following Huh7.5 infection (mean ± SD; n = 3). Statistical significances (***p<0.001, ns non-significant) were determined for each experimental condition versus control condition (100%). (D). Propagation of HCVcc viral particles in Huh7.5-BLd-tm cell cultures. Huh7.5 and Huh7.5 BLd-tm cells were infected with HCVcc H77/JFH-1 at a m.o.i. of 0.1. At day, 1, 3 and 5 post-infection, cell culture supernatants were harvested and used to infect naïve Huh7.5 cells. Viral titers of secondary infection were determined by NS5A immunostaining four days post-infection (mean ± SD; n = 3). (E) BLd-tm inhibits cell entry. Non-transduced Huh7.5 and Huh7.5-BLd-tm were infected with HCVpp H77 and VSVpp. 72h post infection, amount of GFP positive cells were quantified. Percentage of infection of Huh7.5-BLd-tm is normalized for each type of particle on the percentage of infection of non-transduced Huh7.5 (mean ± SD; n = 3). Statistical significances (**p<0.01, ns non-significant) were determined for each experimental condition versus control condition (100%). (F) Soluble E2 binding to Huh7.5-BLd-tm cells. Different doses of soluble E2 were mixed with different concentrations of Huh7.5 and Huh7.5-BLd-tm cells and E2 binding was quantified by flow cytometry. Results represent E2 ability to bind Huh7.5-BLd-tm cells for each condition relatively to the basal E2 ability to bind Huh7.5 cells (determined as 0% binding) for the same condition (mean ± SD; n = 3). *p<0.05. (G) Interaction between BLd-H77 and sE2 detected by ELISA. Different amounts of mouse IgG isotype, AR3B and BLd-H77 were coated overnight into 96-well plates. Coated peptides and antibodies were then incubated with 10ng of soluble E2 (sE2) or not. After washing, soluble E2 was detected using the rat anti-E2 antibody 3/11 and an anti-rat HRP antibody. After measurement of the optical density (O.D.) at 450nm, relative E2 binding was determined by calculating the ratio of O.D. between condition with 10ng of sE2 and no sE2, for each coating condition (mean ± SD; n = 3). **p<0.01, ***p<0.001, ****p<0.0001, ns non-significant. (H) BLd-H77 does not affect HCVcc binding. JC1 HCVcc particles were pre-incubated with BLd-H77 (35 μg/ml), Heparine (250 μg/ml) or PBS and mixed with Huh7.5 cells for 2h at 4. After washing, amounts of cell-associated viral particles were determined by RT-qPCR (mean ± SD; n = 3). Data are shown as percentage of binding, according to binding of HCVcc particles in control condition (PBS). Statistical significances (*p<0.05, ns non-significant) were determined for each experimental condition versus control condition (100%). (I) BLd-H77 can inhibit HCV entry following particle binding. HCVpp particles (HCVpp-H77) were incubated with Huh7.5 in presence of BLd-H77 (50μg/ml) or AR4A (25 μg/ml) during binding (1h at 4°C; 2), entry (4h at 37°C following binding; 3), or following entry (72h at 37°C following media change; 4). GFP levels were quantified 72h post infection. Huh7.5 infected in a similar manner but non-treated with BLd-H77 or AR4A were used as control (1). Percentages of infection were calculated based on viral titer obtained from control conditions. (mean ± SD; n = 3). Statistical significances (***p<0.001, ns non-significant) were determined for each experimental condition versus control condition (100%). (J) Effect of BLd-H77 on cell-cell fusion. LTR-luciferase-transduced 293T cells expressing HCV-H77 E1E2 or VSVG glycoproteins were co-cultivated with Tat-expressing Huh7.5 cells. Following pre-incubation with PBS or with 50μg/ml of BLd-H77, co-cultured cells were exposed to an acid shock (pH5) or not (pH7) and luciferase activities were determined 72h post-exposure. Percentage of fusion of HCV and VSVG glycoproteins at pH5 between control (PBS) or BLd-H77 are indicated (mean ± SD; n = 3). Statistical significances (**p<0.01, ***p<0.001) were determined for each experimental condition versus control condition (100%). (K) Effect of BLd-H77 on virus-liposome fusion assays. H77 HCVpp particles were pre-incubated with different dose of BLd-H77 (50μg/ml, green; 100 μg/ml, blue; 150 μg/ml, red) or not (PBS, black), and mixed with R18-labelled liposomes. Dequenching of R18 was quantified following sample acidification (pH5). Data are represented as non-linear polynomial fitted curves for each experimental condition and display the evolution of the fusion rate (%) over time. Curves are representative of three independent experiments.