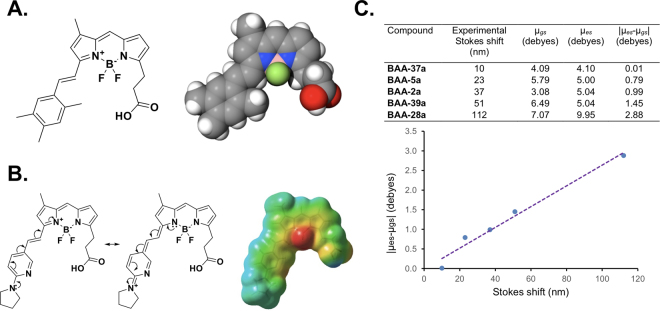
Figure 6.
Chemical Mechanism of BODIPY-based library Stokes shift. (A) Compound BAA-16a did not have heteroatoms in its styryl extended π-system. The relatively long Stokes shift of 58 nm was attributed mainly to the bulky methyl substituents on the styryl extension of this fluorophore. (B) The resonance structures of BAA-39a and its molecular electrostatic potential surface are shown. The nitrogen in the pyrrolidine ring could share its lone pair electrons through the π-conjugated system, while the nitrogen in the pyridine ring could not. The negative charge (red) was localized between the boron and the fluorine atoms, while the positive charge (blue) was distributed along the π-conjugated system. (C) Correlation of the absolute value of the difference in dipole moments between the excited and ground states (|μes-μgs|) vs. the Stokes shift showed a linear relationship in the BAA library. The optimized geometries for the ground state (gs) and excited state (es) were obtained from DFT calculations using the B3LYP hybrid density functional with the 6–31 G(d,p) basis set.