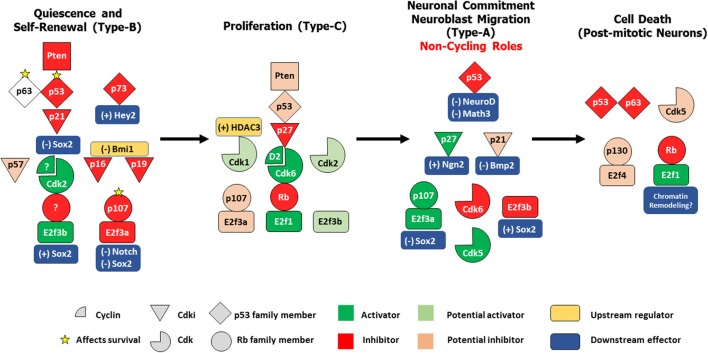
Figure 1.
Differential control of distinct stages in adult SVZ-OB neurogenesis by different cell cycle regulatory proteins. In response to the proper cues, quiescent type-B stem cells get activated to produce rapidly dividing type-C progenitors, which in turn preferentially commit to a neuronal lineage as neuroblasts that migrates along the RMS and differentiate into post-mitotic interneurons in the OB. These four populations are under the tight control of the cell cycle machinery. In both NSCs/type-B cells and NPCs/type-C cells, a classical/general signaling pathway can be constructed based on existing literature: Pten -> p53 -> Cdki -> Cdk/Cyclin -> Rb family member/E2f. However, distinct members of these protein families seem to play non-redundant roles during these two stages e.g., p21, Cdk2, and p107 act specifically in type-B maintenance while p27, Cdk6, and Rb preferentially regulate type-C proliferation. Interestingly, within the same population, family members can have non-overlapping functions e.g., p63 and p73 in controlling survival of type B cells, or even more, might have opposing roles e.g., E2f3a and E2f3b in regulating Sox2-dependent expansion of the NSCs pool. In neuroblasts, several cell-cycle proteins switch to non-cycling tasks as they act to maintain neuronal commitment e.g., p21 and Cdk6 and neuroblast migration e.g., Cdk5. Finally, following neuronal maturation, specific regulators e.g., Rb, p53, Cdk5 act to preserve cell cycle suppression by inhibiting cell-cycle re-entry (CCE), a process prone to deregulation in the aging OB, thus potentially leading to neurodegeneration. (+); positive control, (–); negative control.