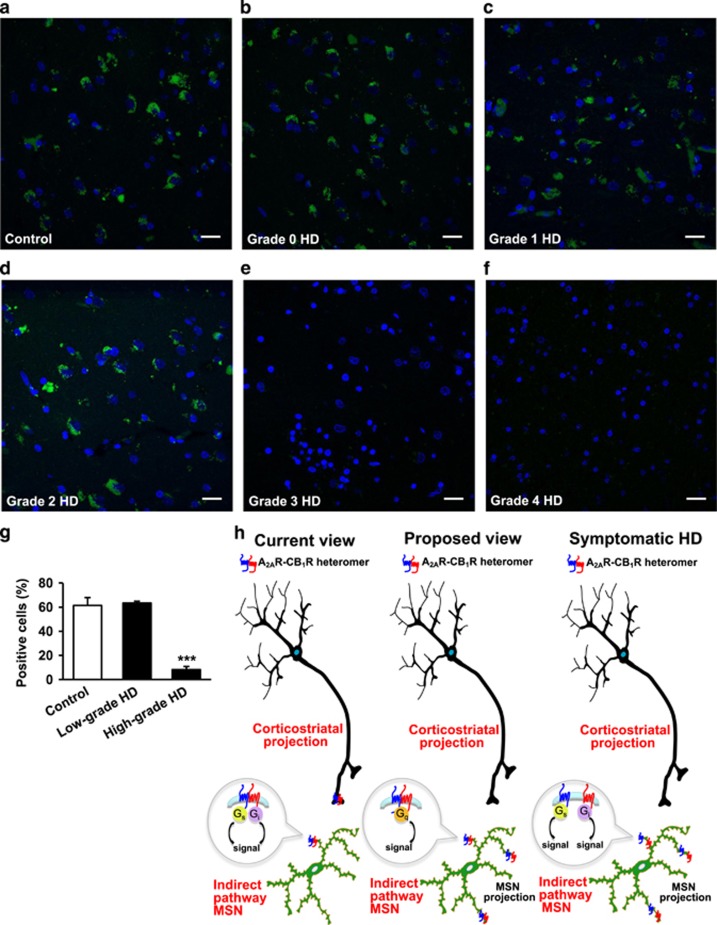
Figure 5.
A2AR-CB1R heteromers are lost in the caudate-putamen of high-grade HD patients. PLA assays were performed in caudate-putamen sections of post mortem samples from control subjects (a) and HD patients at different grades (b–f). A2AR-CB1R heteromers are shown as green dots. Nuclei are colored in blue by DAPI staining. Representative pictures are shown. Scale bar: 20 μm. (g) Quantification of the number of cells containing one or more dots expressed as the percentage of the total number of cells (blue nuclei). Data are the mean±SEM of counts in 21–43 different fields from five control subjects, five low-grade HD patients (1 grade 0, 2 grade 1, plus 2 grade 2) and five high-grade HD patients (2 grade 3, plus 3 grade 4). The characteristics of these human samples are shown in Supplementary Table S1. One-way ANOVA followed by Dunnet post hoc test showed a significant (***p<0.001) decrease of heteromer expression compared to control subjects. Further details of statistical analyses are given in Supplementary Table S2. (h) Scheme depicting the proposed location and G protein-coupling of the A2AR-CB1R heteromer in the dorsal striatum. It is currently believed (left) that the A2AR-CB1R heteromer is located on corticostriatal projections as well as on the somatodendritic compartment of indirect-pathway MSNs. Each protomer would maintain its canonical G protein coupling (Gs for A2AR, and Gi for CB1R). In this study we propose (middle) that the A2AR-CB1R heteromer is located mostly on indirect-pathway MSNs, not only on their somatodendritic compartment but also likely on their terminals. According to our data, the A2AR-CB1R heteromer would facilitate Gq rather than Gs or Gi coupling. In symptomatic HD (right), the A2AR-CB1R heteromer would be disrupted into its constituting protomers.