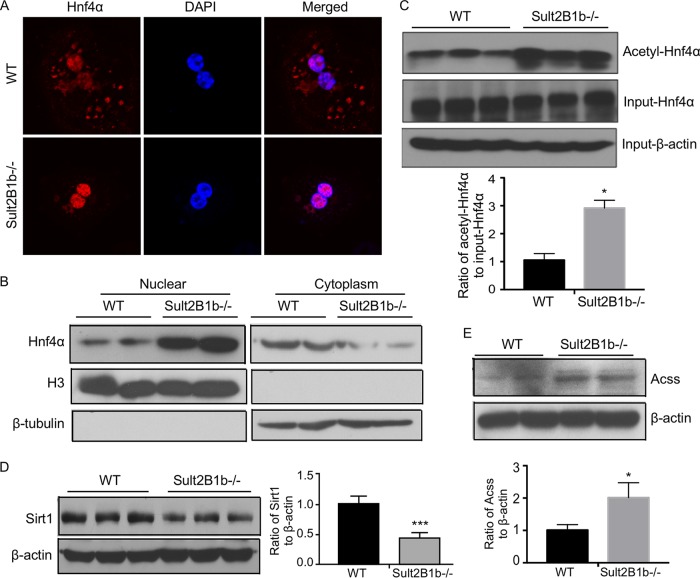
FIG 6.
Ablation of Sult2B1b increases the acetylation of Hnf4α by suppressing the Hnf4α deacetylase Sirt1. Eight-week-old WT and Sult2B1b−/− male mice were used. (A) The subcellular distribution of Hnf4α was visualized by immunofluorescence using an anti-Hnf4α antibody (red) in primary hepatocytes isolated from WT and Sult2B1b−/− mice. DAPI (4′,6-diamidino-2-phenylindole; blue) was used for nuclear counterstaining. (B) Western blot analysis of the Hnf4α protein levels in the nuclear and cytoplasmic fractions of WT and Sult2B1b−/− mouse liver. The purity of the nuclear and cytoplasmic fractions was confirmed by immunoblotting with histone H3 antibody (a nuclear marker) and β-tubulin antibody (a cytoplasmic marker), respectively. (C) Liver lysates of WT and Sult2B1b−/− mice were immunoprecipitated with an anti-acetyl lysine antibody before immunoblotting with an anti-Hnf4α antibody. Shown below the blot is the densitometric quantification of the Western blotting results. (D and E) The hepatic expression levels of Sirt1 (D) and Acss (E) proteins were measured by Western blotting. Shown on the right of (D) or below (E) the blot is the densitometric quantification of the Western blotting results. Results are expressed as means ± SD. *, P < 0.05.