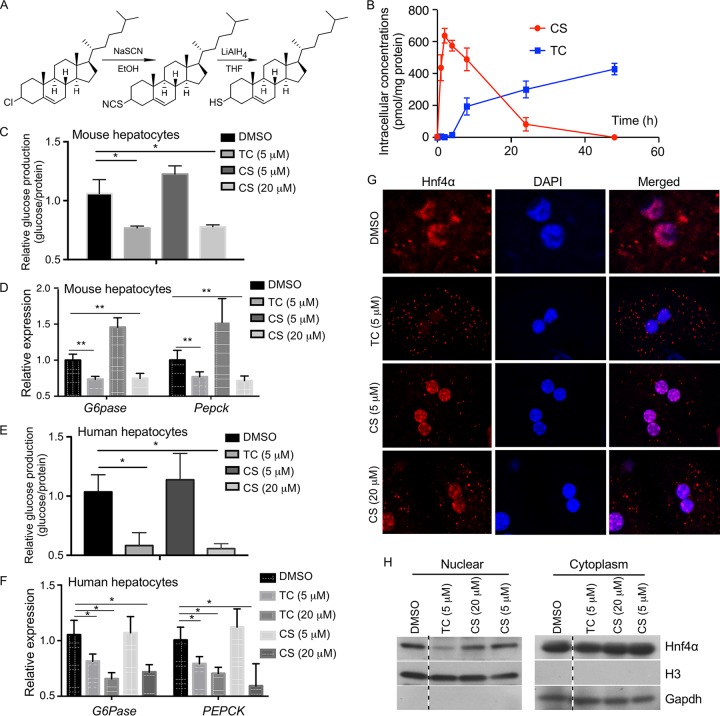
FIG 7.
Thiocholesterol (TC) shows an improved intracellular stability and better efficacy in inhibiting gluconeogenesis in primary hepatocytes. (A) Schematic depiction of the synthesis of TC from cholesteryl chloride. (B) Mouse primary hepatocytes isolated from 8-week-old male WT mice were treated with 5 μM TC or CS for up to 48 h, and the intracellular concentrations of TC and CS were measured by using UPLC-mass spectrometry. (C and D) Glucose production (C) and expression of gluconeogenic genes (D) in mouse primary hepatocytes treated with the indicated concentrations of drugs for 24 h. Cells were treated with 10 μM FSK for 2.5 h before the glucose production assay. The expression of each gene or glucose production was arbitrarily set as 1 in cells treated with DMSO. (E and F) The designs of the experiments were similar to those described for panels C and D, except that human primary hepatocytes were used. (G) The subcellular distribution of Hnf4α was visualized by immunofluorescence using an anti-Hnf4α antibody (red) in primary hepatocytes isolated from WT mice and treated with the indicated concentrations of drugs. DAPI (blue) was used for nuclear counterstaining. (H) The subcellular distribution of Hnf4α was measured by nuclear-cytosolic fractionation of the hepatocytes and Western blotting. Histone H3 and glyceraldehyde-3-phosphate dehydrogenase (Gapdh) are nuclear and cytoplasmic markers, respectively. All four lanes are from the same blot, with the dotted lines indicating noncontinuous lanes. Results are expressed as means ± SD. *, P < 0.05; **, P < 0.01.