Abstract
Background
The relationship between competitive sports and atrial fibrillation (AF) is controversial. We aimed to systematically evaluate and summarize all published observational data on the association between competitive sports and AF.
Methods and results
We searched PubMed, EMBASE, Scopus and SportDiskus for all observational studies that assessed the risk of AF among athletes involved in competitive sports. Data were extracted and pooled odds ratios (OR) were calculated using random effects models. Six cohort studies and 2 case-control studies with a total of 9113 subjects were included in our meta-analysis. Pooled analyses showed an increased risk of incident and prevalent AF among athletes compared to the general population (OR = 1.64 [95% confidence interval (CI): 1.10–2.43]). Age-stratified analysis revealed an effect modification with age. Studies enrolling younger adults (<54 years) had an increased risk of AF among athletes compared to controls (OR = 1.96 [95% CI: 1.06–3.65]), but this association was not seen among older adults ≥54 years (OR = 1.41 [95% CI: 0.81–2.44], p = 0.23).
Conclusion
Athletes have an increased risk of AF compared to the general population. Age appears to modify the risk of AF in athletes.
Keywords: Atrial fibrillation, Athletes, Meta-analysis
1. Background
Atrial fibrillation (AF) is the most common cardiac rhythm disturbance, affecting between 2.7 and 6.1 million Americans [1]. The prevalence of AF increases from 2% in the young and middle aged to 9% in people older than 65 years [2]. AF increases the risk of stroke 5-fold and contributes to an estimated 130,000 deaths annually [1].
Large studies have shown that exercise has a U-shaped relationship with atrial fibrillation among the general population. The risk of atrial fibrillation decreases with moderate exercise but increases at the both ends of the exercise spectrum [[3], [4], [5]]. Nonetheless, the relationship between competitive sports and AF is controversial. A meta-analysis of small observation studies totaling 655 athletes and 895 controls showed significantly increased odds of AF in athletes compared to the general population (OR 5.29; 95% CI 3.57–7.85) [6]. However, Pelliccia et al. did not find an increased prevalence of AF among 1777 athletes without structural heart disease [7].
In light of more recent observational studies, we aim to update the findings of prior meta-analyses that have evaluated the risk of AF in athletes. Understanding the association between competitive sports participation and AF would contribute evidence to the growing field of cardiovascular care of young and middle aged athletes.
2. Methods
2.1. Search strategy
We searched Pubmed, Embase, Scopus and SportDiskus for all relevant full text articles published before August 1, 2017, without language restriction. The search keywords used in PubMed were (“atrial fibrillation” OR “auricular fibrillation”) AND (“endurance” OR “exercise” OR “sports” OR “athletes”). The same search was adapted for Embase, Scopus and SportDiskus. References of retrieved articles were also reviewed for relevant articles.
2.2. Study eligibility
Articles initially retrieved by systematic search were screened by title. The following criteria were used to retain articles for further review: (1) Study assessed the association between competitive or semi-competitive sports and atrial fibrillation. (2) Study was a case-control or cohort study. (3) Study provided odds ratio and confidence intervals or the odds ratio could be calculated from the data provided.
We excluded studies using the following criteria: (1) Study evaluated physical activity that was not related to competitive or semi-competitive sports. (2) Study focused on valvular or postoperative atrial fibrillation. (3) Study focused on atrial flutter. When studies included both atrial flutter and atrial fibrillation, we extracted only the atrial fibrillation numbers for use in our analysis. (4) Study had control arm that was not representative of the general population. (5) Study was a case report, letter to editor or comment. (6) Study did not have sufficient data.
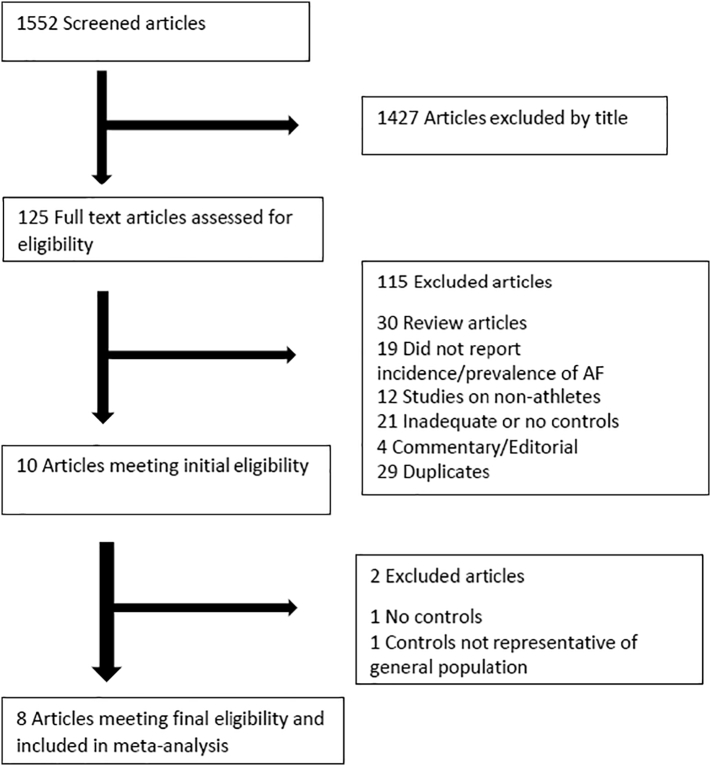
Our initial search retrieved 1552 articles. See Fig. 1 for PRISMA flow diagram. After exclusion by title, 125 articles were selected for detailed review. One hundred and fifteen (115) articles were further excluded. Of these, 30 were review articles, 19 did not report on incidence or prevalence of atrial fibrillation, 12 studies involved non-athletes as cases, 21 studies had inadequate or no controls, 4 were editorials or commentaries, and 29 were duplicates. Two studies were later excluded because one had controls not representative of the general population, and the other did not have controls. Eight (8) studies were included in the meta-analysis.
Fig. 1.
PRISMA flow chart describing study selection.
2.3. Data extraction and quality assessment
Two reviewers (HA and VC) extracted all data. Data extracted include study location, sample size, baseline demographic and clinical data, type of sport, criteria for AF diagnosis, counts for AF and controls, and adjusted/unadjusted odds ratios (OR) and confidence intervals (CI). Differences in data between the 2 reviewers were resolved by a 3rd reviewer. Study quality was assessed by the Newcastle Ottawa Scale [8]. This assessed three domains, including risk of bias in selection, comparability and exposure assessment. We classified studies with quality scores of 6 or greater as high quality (maximum score on the scale is 9). Otherwise, studies were classified as low quality.
2.4. Definition of atrial fibrillation
Four of the selected studies assessed lone AF. This was defined as atrial fibrillation in the absence of structural heart disease or identifiable etiology such as hyperthyroidism, diabetes, hypertension, etc. [1]. The remainder of the selected studies (with the exception of one) did not specify AF type, but controlled for known AF risk factors.
2.5. Data synthesis and analysis
We extracted OR and 95% CI, or calculated them from raw outcome data obtained from the studies. Microsoft Excel 2013 and Review Manager 5.3 (RevMan) [9] were used for pooled analysis. We calculated the logarithm of the OR and standard error of its variance. The generic inverse variance method with random effects model [10] was used for pooled analysis. The presence of heterogeneity was discerned by Chi Square tests as described by Woolf [11]. The degree of heterogeneity was measured by the I2 statistic. Significant heterogeneity was determined as I2 > 40% [12]. Publication bias was visually assessed using funnel plot diagrams. We planned to perform stratified analysis based on study quality, gender and mean age of the population. However, the number of women in the studies was too small to allow for stratification by gender.
Our study was performed in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA) [13].
3. Results
Eight studies with a total sample size of 9113 were included in our analysis (Table 1) [[14], [15], [16], [17], [18], [19], [20], [21]]. Six studies were cohort studies while 2 were case-control studies. Five of the studies included male subjects only. Two studies (Heidbuchel et al. and Calvo et al.) included 83.2% and 87% men respectively, while one study (Myrstad et al.) did not report on gender. Five of the studies included participants of individual sports (cycling, skiing, marathon running, orienteering), while 3 studies included team sports. Four studies (Elosua et al., Calvo et al., Molina et al. and Karjaleinen et al.) included only patients with lone AF. The other four studies included all AF with no specific exclusions (Table 1). Four studies adjusted for varying confounders as shown in Table 1.
Table 1.
Characteristics of included studies.
| First author | Year | Location | Type of sport | Design | Mean age of athletes (years) | Male (%) | Quality assessment (average of 2 scores) |
Variables adjusted for in analysis | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk of selection bias | Comparability | Exposure assessment | Quality score | ||||||||
| Karjalainen et al. | 1998 | Finland | Orienteering | Cohort study | 47.5 | 100 | 3 | 2 | 2 | 7 | None |
| Heidbuchel et al. | 2006 | Leuven, Belgium | Mixed | Cohort study | 53 | 83.2 | 3 | 1 | 2 | 6 | Age, pre-procedural AF, history of cardioversion, number of anti-arrhythmics pre-ablation, ACE/ARB/diuretics, continued endurance sports practice |
| Elosua et al. | 2006 | Barcelona, Spain | Mixed | Case-control study | 41.7 | 100 | 2 | 2 | 1.5 | 5.5 | None |
| Baldesberger et al. | 2008 | Switzerland | Cycling | Cohort study | 66 | 100 | 3 | 2 | 2 | 7 | Age, BMI, hypertension, current level of activity |
| Calvo et al. | 2016 | Barcelona, Spain | Mixed | Case-control study | 46 | 87 | 3.5 | 1 | 2 | 6.5 | None |
| Molina et al. | 2008 | Barcelona, Spain | Marathon running | Cohort study | 39 | 100 | 3 | 2 | 2 | 7 | Age, systolic blood pressure |
| Myrstad et al. | 2014 | Norway | Skiing | Cohort study | 68.9 | 100 | 3 | 2 | 0.5 | 5.5 | Coronary artery disease, hypertension, DM, age, height, BMI, education level, alcohol consumption, smoking, leisure time physical activity |
| Myrstad et al. | 2016 | Norway | Skiing | Cohort study | 68.5 | Unstated | 3 | 0 | 2 | 5 | None |
AF: atrial fibrillation, ACE: angiotensin converting enzyme inhibitor, ARB: angiotensin receptor blocker, DM: diabetes mellitus, BMI: body mass index.
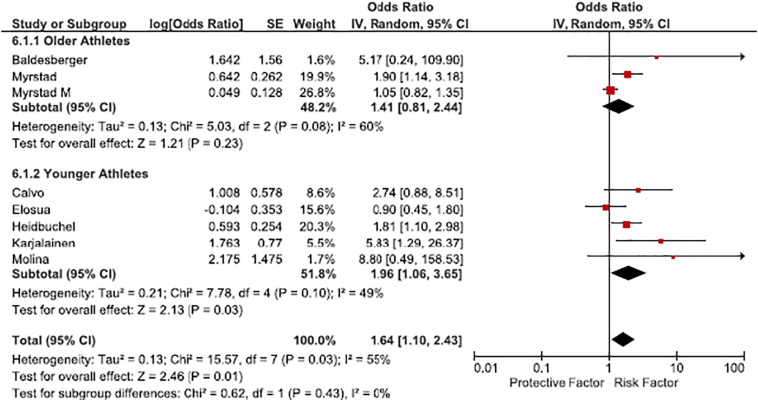
The pooled analysis using a random effects model showed that athletes had a 64% higher odds of incident and prevalent AF compared to the general population (OR 1.64 [95% CI 1.10–2.43]) (Fig. 2). There was significant heterogeneity between studies (Chi2 = 15.57, p = 0.03, I2 = 55%). Thus, we performed stratified analysis by study quality and age to improve the accuracy of our risk measure.
Fig. 2.
Risk of atrial fibrillation in athletes compared to the general population, stratified by age of enrolled athletes.
3.1. Stratified analysis
3.1.1. Quality
Five studies met criteria for higher quality (Newcastle Ottawa Quality score ≥ 6) while 3 studies were judged as lower quality (Table 1). This stratification led to homogeneity among the higher quality studies. Among high quality studies, athletes had significantly higher risk of AF (OR 2.23 [95% CI 1.45–3.41], I2 = 0%) while there was no significant difference between athletes and controls in low quality studies (OR 1.22 [95% CI 0.81–1.83], I2 = 57%).
3.1.2. Age
Age appeared to be an effect modifier when studies were dichotomized by age at 54 years (Fig. 2). Younger athletes (age < 54 years) had approximately 2 times increased odds of AF compared to the controls of similar age distribution (OR 1.96 [95% CI 1.06–3.65], I2 = 49%). Conversely, the risk of AF was not significantly increased in older athletes compared to the general population of similar age (OR 1.41 [95% CI 0.81–2.44], I2 = 60%). However, the studies included in this stratified analysis were heterogeneous.
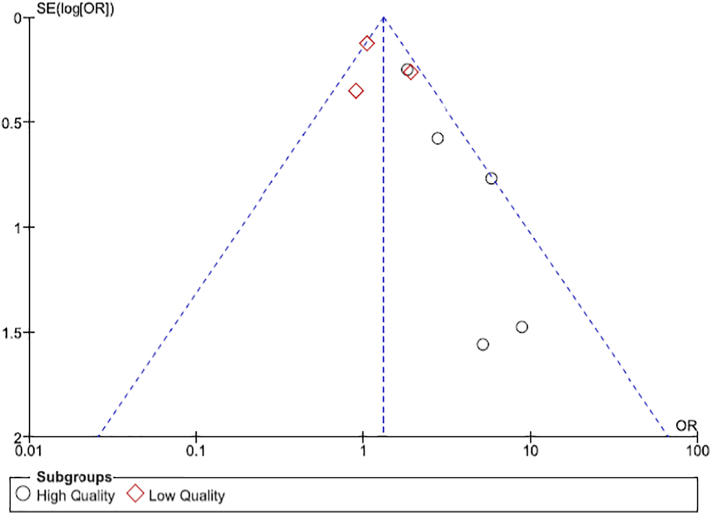
The funnel plot of all studies was asymmetric, suggesting publication bias (Fig. 3). It is possible that negative or null studies were not published.
Fig. 3.
Funnel Plot showing evidence of publication bias.
4. Discussion
We found that athletes have increased risk of AF compared to the general population. There appears to be significant interaction with age. Younger athletes (age < 54) have significantly increased odds of AF compared to controls (OR 1.96 [1.06–3.65]). However, the association between AF and competitive sports is not significant in older individuals ≥54 years of age (OR 1.41 [0.81–2.44]).
Our findings are similar to a meta-analysis published in 2009 by Abdulla and Nielsen [6]. They included 6 observational studies comparing athletes and the general population and found an increased risk of AF (OR 5.29 [95% CI: 3.57–7.85]). However, our effect size was much smaller, likely due to our inclusion of larger studies that were published since the publication of that meta-analysis. Furthermore, the average age of the population in that meta-analysis was 51 years, and they did not assess for age interaction.
In a meta-analysis by Brunetti et al. that included all levels of physical activity [22], age was found to modulate the relationship between physical exercise and AF. Specifically, there was an increased risk of AF in studies enrolling subjects <54 years (OR 5.30 [3.48–8.20]). However, there was an inverse association in studies enrolling subjects >54 years (OR = 0.84 [0.76–0.92]). We found a similar trend in our meta-analysis. Athletes younger than 54 years had a significantly increased risk of AF, but the statistical significance was lost in athletes ≥54 years of age. Since AF is strongly associated with age, it stands to reason that sports predisposes participants to earlier onset AF but there is no increase in risk in older athletes compared to non-athletes of similar age.
The mechanism by which competitive sports predisposes athletes to AF is still a matter of intense speculation. It has been proposed that AF initiation and perpetuation occur due to an interplay of triggers, modulators, and an appropriate substrate [23,24]. Excessive parasympathetic and sympathetic stimulation, such as occurs in athletes, can trigger atrial ectopy and shorten action potential duration, thereby initiating and sustaining AF [25,26]. Chronic pressure and volume overload from increased cardiac output leads to adverse anatomic and electrophysiologic remodeling, characterized by biatrial dilation, inflammation and myonecrosis, fibrosis and non-homogenous conduction [[23], [24], [25], [26], [27], [28], [29]]. Conversely, moderate exercise has been shown to be protective against remodeling [30]. It appears that each of the triggers and substrates are necessary but not sufficient causes for AF. For example, it is unclear if increased atrial ectopy by itself leads to more AF in athletes [24]. Furthermore, Guasch et al. [29] showed that exercise increased AF susceptibility, vagal tone, atrial enlargement and fibrosis in mice that exercised for 16 weeks. However, after detraining for 4 weeks, AF susceptibility and heightened vagal tone resolved while LA dilation and fibrosis persisted [29]. Thus, the interaction between multiple factors including extreme autonomic input, electrophysiologic changes and anatomic remodeling is necessary for AF initiation and maintenance in athletes.
4.1. Limitations
The asymmetry of our funnel plot suggested evidence of publication bias. It is likely that negative or null studies were not published. Moreover, we did not search for fugitive literature. Thus, it is possible that our risk estimates may be overestimated.
There was significant heterogeneity among the included studies. Potential causes were different study designs (case-control and cohort studies) and different methods of AF ascertainment and assessment of exposure. Also, studies did not uniformly adjust for confounders. We used stratified analysis to determine the reasons for some of the heterogeneity, but more high quality studies should be performed on this topic.
We cannot extrapolate our findings to female athletes as they were only a small minority of the included population. To date, it remains unclear whether female athletes are at an increased risk of AF. Furthermore, we included all types of competitive sports but it is possible that the risk of AF varies with the different sports. Since most of the studies included endurance sports such as marathon running, cycling and skiing, these findings may not be generalizable to all competitive sports.
5. Conclusion and future studies
Athletes have increased risk of AF compared to the general population. The increased risk was only statistically significant in analyses of young athletes. Further, our findings may be specific to male athletes only as few female athletes were included in our study.
Large rigorous observational studies assessing AF risk in various sports are needed. Female athletes make up a significant proportion of sports participants, and they should be included in future studies. Future studies should also evaluate the prognosis of athletes who develop early onset AF to determine whether they at increased risk of stroke or mortality.
Funding source
None.
Disclosures
The authors report no relationships that could be construed as a conflict of interest.
Acknowledgements
The authors would like thank Kayla Heslin of the College of Public Health at the University of Iowa for her suggestions in the data extraction process.
References
- 1.January C.T., Wann L.S., Alpert J.S. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. J. Am. Coll. Cardiol. 2014;64(21):2246–2280. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 2.McManus D.D., Rienstra M., Benjamin E.J. An update on the prognosis of patients with atrial fibrillation. Circulation. 2012;126:e143–e146. doi: 10.1161/CIRCULATIONAHA.112.129759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mozaffarian D., Furberg C., Psaty B., Siscovick D. Physical activity and incidence of atrial fibrillation in older adults: the cardiovascular health study. Circulation. 2008;118:800–807. doi: 10.1161/CIRCULATIONAHA.108.785626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aizer A., Gaziano J.M., Cook N.R., Manson J.E., Buring J.E., Albert C.M. Relation of vigorous exercise to risk of atrial fibrillation. Am. J. Cardiol. 2009;103:1572–1577. doi: 10.1016/j.amjcard.2009.01.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohanty S., Mohanty P., Tamaki M. Differential association of exercise intensity with risk of atrial fibrillation in men and women: evidence from a meta-analysis. J. Cardiovasc. Electrophysiol. 2016 Sep;27(9):1021–1029. doi: 10.1111/jce.13023. [DOI] [PubMed] [Google Scholar]
- 6.Abdulla J., Nielsen J.R. Is the risk of atrial fibrillation higher in athletes than in the general population? A systematic review and meta-analysis. Europace. 2009;11:1156–1159. doi: 10.1093/europace/eup197. [DOI] [PubMed] [Google Scholar]
- 7.Pelliccia A., Maron B.J., Di Paolo F.M. J. Am. Coll. Cardiol. Aug 16 2005;46(4):690–696. doi: 10.1016/j.jacc.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 8.Wells G.A., Shea B., O'Connell D. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses. http://www.ohri.ca/programs/clinicalepidemiology/oxford.htm Available from:
- 9.Review Manager (RevMan) The Nordic Cochrane Center, The Cochrane Collaboration; Copenhagen: 2014. [Computer Program]. Version 5.3. [Google Scholar]
- 10.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. Fixed and Random Effects Models. [DOI] [PubMed] [Google Scholar]
- 11.Woolf B. On estimating the relation between blood group and disease. Ann. Hum. Genet. 1955;19:251–253. doi: 10.1111/j.1469-1809.1955.tb01348.x. Heterogeneity test. [DOI] [PubMed] [Google Scholar]
- 12.Ryan R., Cochrane Consumers and Communication Review Group Heterogeneity and Subgroup Analyses in Cochrane Consumers and Communication Group Reviews: Planning the Analysis at Protocol Stage. December 2016. http://cccrg.cochrane.org (accessed date)
- 13.Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement Ann. Intern. Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 14.Karjalainen J., Kujala U.M., Kaprio J., Sarna S., Viitasalo M. Lone atrial fibrillation in vigorously exercising middle aged men: case-control study. BMJ. 1998;316(7147):1784–1785. doi: 10.1136/bmj.316.7147.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heidbuchel H., Anne W., Willems R., Adriaenssens B., Van de Warf F., Ector H. Endurance sports id a risk factor for atrial fibrillation after ablation for atrial flutter. Int. J. Cardiol. 2006;107:67–72. doi: 10.1016/j.ijcard.2005.02.043. [DOI] [PubMed] [Google Scholar]
- 16.Elosua R., Arquer A., Mont L. Sport practice and the risk of lone atrial fibrillation: a case-control study. Int. J. Cardiol. 2006;108:332–337. doi: 10.1016/j.ijcard.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 17.Baldesberger S., Bauersfeld U., Candinas R. Sinus node disease and arrhythmias in the long-term follow-up of former professional cyclists. Eur. Heart J. 2008;29(1):71–78. doi: 10.1093/eurheartj/ehm555. [DOI] [PubMed] [Google Scholar]
- 18.Calvo N., Ramos P., Montserrat S. Emerging risk factors and the dose-response relationship between physical activity and lone atrial fibrillation: a prospective case-control study. Europace. 2016;18:57–63. doi: 10.1093/europace/euv216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molina L., Mont L., Marrugat J. Long-term endurance sport practice increases the incidence of lone atrial fibrillation in men: a follow-up study. Europace. 2008;10:618–623. doi: 10.1093/europace/eun071. [DOI] [PubMed] [Google Scholar]
- 20.Mysrtad M., Lochen M.-L., Graff-Iversen S. Increased risk of atrial fibrillation among elderly Norwegian with a history of long-term endurance sport practice. Scand. J. Med. Sci. Sports. 2014;24:e238–e244. doi: 10.1111/sms.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myrstad M., Aaronaes M., Graff-Iversen S., Ariansen I., Nystad W., Ranhoff A.H. Physical activity, symptoms, medication and subjective health among veteran endurance athletes with atrial fibrillation. Clin. Res. Cardiol. 2016;105:154–161. doi: 10.1007/s00392-015-0898-0. [DOI] [PubMed] [Google Scholar]
- 22.Brunetti N.D., Santoro F., Correale M. Incidence of atrial fibrillation is associated with age and gender in subjects practicing physical exercise: a meta-analysis and meta-regression analysis. Int. J. Cardiol. 2016;221:1056–1060. doi: 10.1016/j.ijcard.2016.07.133. [DOI] [PubMed] [Google Scholar]
- 23.Olshansky B., Sullivan R. Increased prevalence of atrial fibrillation in the endurance athlete: potential mechanisms and sport specificity. Phys. Sportsmed. 2014;42(1):45–51. doi: 10.3810/psm.2014.02.2047. [DOI] [PubMed] [Google Scholar]
- 24.D'Ascenzi F., Cameli M., Ciccone M. The controversial relationship between exercise and atrial fibrillation: clinical studies and pathophysiologic mechanisms. J. Cardiovasc. Med. 2015;16:802–810. doi: 10.2459/JCM.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 25.Patterson E., Po S.S., Scherlag B.J., Lazzara R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm. 2005;2(6):624–631. doi: 10.1016/j.hrthm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Nattel S., Burstein B., Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ. Arrhythm. Electrophysiol. 2008;1(1):62–73. doi: 10.1161/CIRCEP.107.754564. [DOI] [PubMed] [Google Scholar]
- 27.Frustaci A., Cltlmenti C., Bellocci F., Morgante E., Russo M.A., Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180–1184. doi: 10.1161/01.cir.96.4.1180. [DOI] [PubMed] [Google Scholar]
- 28.Masci G., Perrotta L., Galanti G., Padeletti L. Atrial fibrillation in athletes. Int. J. Sports Med. 2013;34:379–384. doi: 10.1055/s-0032-1321896. [DOI] [PubMed] [Google Scholar]
- 29.Guasch E., Benito B., Qi X. Atrial fibrillation promotion by endurance exercise. Demonstration and mechanistic exploration in an animal model. J. Am. Coll. Cardiol. 2013;62(1):68–77. doi: 10.1016/j.jacc.2013.01.091. [DOI] [PubMed] [Google Scholar]
- 30.Lachance D., Plante E., Bouchard-Thomassin A.A. Moderate exercise training improves survival and ventricular remodeling in an animal model of left ventricular volume overload. Circ. Heart Fail. 2009;2:437–445. doi: 10.1161/CIRCHEARTFAILURE.108.845487. [DOI] [PubMed] [Google Scholar]