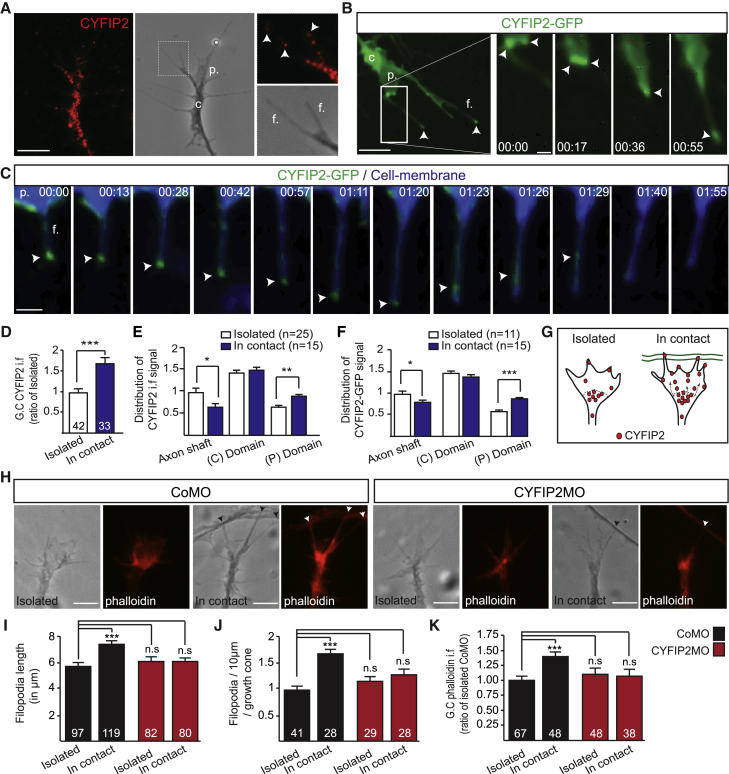
Figure 4.
CYFIP2 Regulates Filopodial Dynamics and F-actin in the Growth Cone Peripheral Domain upon Axon-Axon Contact
(A) CYFIP2 immunostaining on stage 32 Xenopus retinal growth cone (GC). Arrowheads indicate CYFIP2 in filopodia.
(B) Time-lapse imaging of CYFIP2-GFP movements in RGC GC. Arrowheads indicate the accumulation of CYFIP2-GFP.
(C) Time-lapse imaging of CYFIP2-GFP movements in an elongating GC filopodia labeled by a membrane marker (blue). Arrowheads indicate CYFIP2-GFP accumulation.
(D) Quantification of CYFIP2 signal intensity in GC.
(E) Distribution of CYFIP2 signal intensity along RGC axon shaft (last 10 μm) and GC central (C) and peripheral (P) domains.
(F) Distribution of CYFIP2-GFP signal intensity along RGC axon shaft (last 10 μm) and GC central (C) and peripheral (P) domains.
(G) Scheme illustrating the observed relocalization of CYFIP2 in the GC peripheral domain during axon-contact.
(H) Phalloidin immunostaining on isolated or in contact stage 32 Xenopus retinal GCs from CoMO- and CYFIP2MO-injected embryos.
(I and J) Quantifications of filopodia length (I) and number (J).
(K) Quantification of phalloidin signal intensity in GC.
The numbers of GC (D, J, and K) or filopodia (I) analyzed are indicated on the bars. Error bars represent SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, n.s., non-significant (Mann-Whitney test for D–F and I–K). The GC central domain (c.), peripheral domain (p.), and filopodia (f.) are indicated (A–C). Time stamps are in the format of min:s. Scale bars: 5 μm (A, B left panel, and H); 1 μm (B right panels); 2 μm (H). See also Figure S2.