Figure 4. MRPs Are Synthesized and Imported in Excess and Can Be Unstable if Not Assembled.
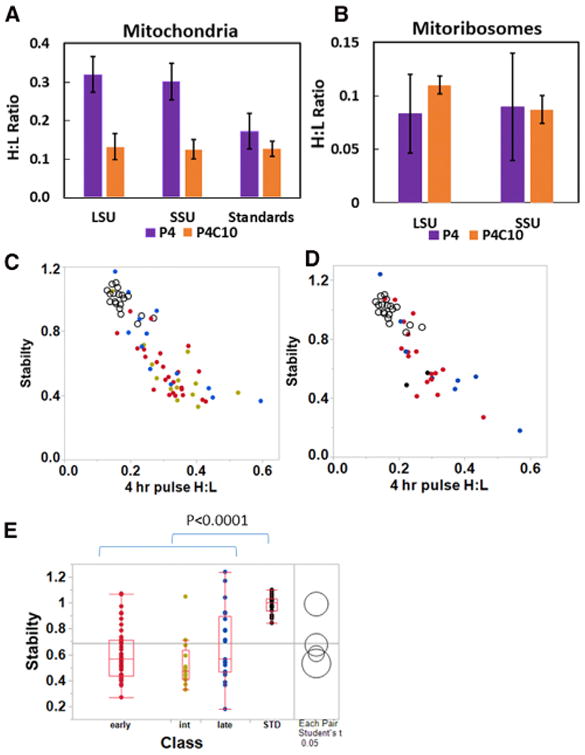
(A) The H:L ratio immediately after 4 hr of pulse labeling is greater for MRPs than for a standard set of mitochondrial proteins, indicating a higher rate of synthesis and mitochondrial import, but the H:L ratio of MRPs declines during a 10-hr chase to a value not significantly different from the stable standard proteins. Mean values are shown ± SD.
(B) The H:L values observed for MRPs in assembled mitoribosomes before and after the chase are shown for comparison. The large SE after the pulse reflects variable assembly kinetics of individual proteins and is significantly smaller after the chase. Note the change in scale of the ordinate compared to (A).
(C and D) The stability of LSU (C) and SSU (D) MRPs in early (red), intermediate (green), and late (blue) assembly classes is plotted against the H:L ratio after the pulse. Standard proteins are shown with open circles in both plots.
(E) The stabilities (P4C10/expected) of early, intermediate, and late assembly classes of MRPs are significantly lower than those of the standard proteins, but not significantly different from one another.
See also Tables S1 and S2.
