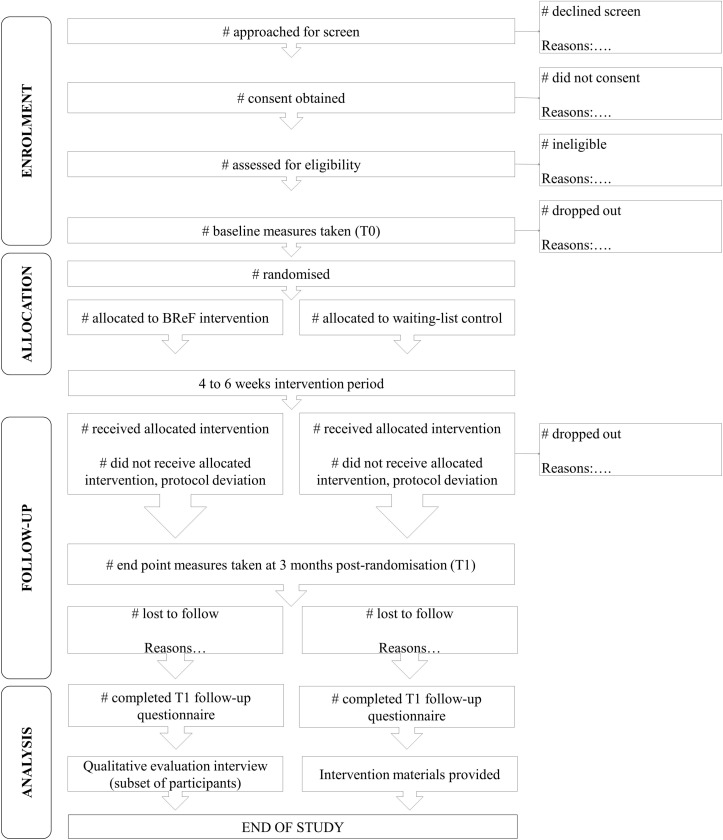
Figure 2.
Anticipated flow of participants through the study. Number of patients approached for screen, those who consented, and those who were assessed for eligibility will be recorded. Eligible patients will be invited to complete a baseline questionnaire (T0). After completion of the baseline questionnaire, participants will be randomised. Participants in the intervention arm will receive the intervention over 4–6 weeks. All participants will complete a follow-up questionnaire at 3 months postrandomisation (T1). Participants in the intervention arm will be invited to take part in a qualitative evaluation interview at the end of their involvement in the study. After completion of the follow-up questionnaire, participants in the control arm will receive the intervention materials.