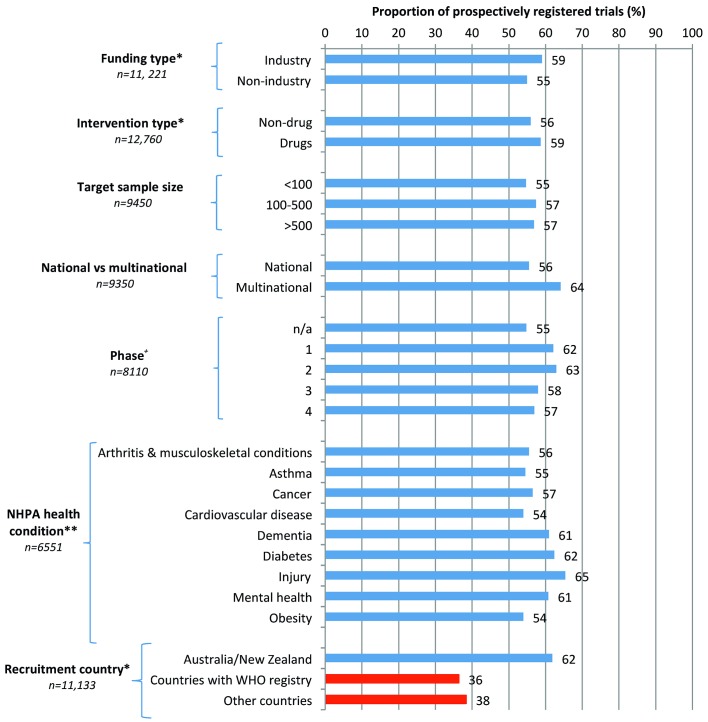
Figure 2.
Prospective registration rates across various study characteristics. *A trial can add >1 entry for these fields. Therefore, the total number of data points is greater than the total number of trials. +Phase is an optional field on the ANZCTR registration form. **By Australian NHPA, which covers 6551/14682 (45%) conditions in included trials. Note that a single trial can select up to three conditions. Proportion of missing values for mandatory fields was small. There were no missing values for health condition and target sample size, 2 (0.02%) for funding type and 55 (0.43%) for intervention type. There were 100 missing values for recruitment country (0.90%) and multinational versus national (1.07%). Almost all missing values are from 2006 to 2007 where data collection procedures were less stringent. ANZCTR, Australian New Zealand Clinical Trials Registry; NHPA, National Health Priority Area.