Abstract
Background:
Glutathione is a key scavenging antioxidant that opposes the proinflammatory signaling of hydrogen peroxide. Boosting cellular glutathione levels may have broad utility in the prevention and treatment of disorders driven by oxidative stress. Supplemental N-acetylcysteine has been employed for this purpose. Could supplemental glycine likewise promote glutathione synthesis?
Methods:
We conducted a review of the pertinent literature using PubMed.
Results:
Tissue glycine levels are lower than the glutathione synthase Michaelis constant (Km) for glycine. When glycine availability is too low to sustain a normal rate of glutathione synthesis, the consequent rise in tissue levels of gamma-glutamylcysteine leads to an increase in urinary excretion of its alternative metabolite 5-L-oxoproline. The fact that urinary excretion of this metabolite is elevated in vegetarians and others consuming relatively low-protein diets strongly suggests that dietary glycine can be rate-limiting for glutathione synthesis in normally fed humans. Moreover, supplemental glycine has been reported to increase tissue glutathione levels in several animal studies. Glycine is a biosynthetic precursor for porphyrins, purines, creatine, sarcosine, and bile salts; is an agonist for glycine-gated chloride channels and a coagonist for N-methyl-D-aspartate receptors; inhibits protein glycation; and increases hepatic production of pyruvate, an effective scavenger of hydrogen peroxide. Supplemental glycine may have the potential for improving endothelial function, preventing cardiac hypertrophy, aiding control of metabolic syndrome, preventing the complications of diabetes, dampening inflammation, protecting the liver, and promoting effective sleep.
Conclusion:
Clinical research is warranted to evaluate the impact of supplemental glycine on glutathione levels and on various health disorders.
Keywords: Acetylcysteine, chloride channels, glucagon, glucagon-like peptide 1, glutathione, glutathione synthase, glycine
INTRODUCTION
Reduced glutathione is one of the two most prominent scavenging antioxidants within cells alongside ascorbate; its concentration is in the low millimolar range. In addition to providing protection from nonspecific oxidant damage and acting as an electron donor for glutathione peroxidase and glutaredoxin, reduced glutathione reverses the prooxidative signaling mediated by hydrogen peroxide that plays a prominent pathogenic role in many health disorders.1-3 Glutathione is also employed in the conjugation and excretion of xenobiotics. Cellular levels of this key antioxidant tend to decline during the aging process.4 Therefore, practical strategies for optimizing tissue glutathione levels are needed.
METHODS
We conducted a review of the literature using PubMed keyword searches and citations in pertinent papers. Keywords were glutathione, glutathione synthase, N-acetylcysteine, glycine, chloride channels, 5-L-oxoproline, and lipoic acid.
DIETARY GLYCINE REGULATION OF GLUTATHIONE SYNTHESIS
Availability of the amino acid cysteine is known to be rate-limiting for the synthesis of glutathione, and it is well documented, both clinically and in animal studies, that cysteine supplementation—most practically achieved by administration of N-acetylcysteine (NAC)—can boost glutathione synthesis and levels.5,6 Dietary glycine levels may likewise modulate glutathione synthesis.
The tripeptide glutathione is synthesized in a 2-step process in which cysteine and glutamate are linked by glutamate cysteine ligase to form gamma-glutamylcysteine. This product is then linked to glycine by glutathione synthase. The Michaelis constant (Km) of human glutathione synthase for glycine has been reported as 0.58 mmol/L7 and 1.34 mmol/L8; why these values are not closer is not clear. A clinical study found that red blood cell concentrations of glycine averaged 487 μmol/L in young subjects and 218 μmol/L in elderly subjects; this level rose to 529 μmol/L in the elderly subjects after they were administered 100 mg/kg of both glycine and NAC daily for 14 days.9 Therefore, intracellular glycine concentrations appear to be no higher than, and possibly well below, the glutathione synthase Km for glycine, and practical glycine supplementation may notably increase intracellular glycine levels. Hence, glycine supplementation could possibly stimulate glutathione synthesis in humans. The expected rise in glutathione levels would likely be somewhat lower than the initial rise in synthesis rate, as glutathione acts as a feedback competitive inhibitor of glutamate cysteine ligase.10 In animal studies, glycine supplementation has been reported to increase tissue glutathione levels in milk-fed piglets and in rats subjected to burn injury, alcohol-induced liver injury, or fructose-induced metabolic syndrome.11-14
Clinical studies evaluating urinary excretion of 5-L-oxoproline provide evidence that dietary glycine is indeed limiting for glutathione synthesis in humans consuming their typical diets (as opposed to experimental diets depleted of glycine).15-17 When glycine availability is too low to sustain normal rates of glutathione synthesis, the consequent rise in tissue levels of gamma-glutamylcysteine results in increased conversion of this compound to 5-L-oxoproline that is then excreted in the urine.15,16 In a study comparing daily urinary excretion of this metabolite in omnivores and vegetarians, vegetarian males excreted 86% more 5-L-oxoproline than omnivore males, and vegetarian females excreted 37% more than omnivore females.17 These researchers also found that urinary excretion of 5-L-oxoproline rose significantly when subjects were switched from a diet providing 6.2 g of nitrogen per day to one providing 4 g of nitrogen per day. These findings strongly suggest that even though glycine is a nonessential amino acid that can be synthesized from other substrates, the amounts of glycine provided by some common diets is limiting for the synthesis of glutathione. Evidently, supplemental glycine should have its largest impact on glutathione levels in those who consume diets relatively low in protein, notably vegetarians and vegans.
These considerations suggest that intakes of cysteine and glycine jointly determine glutathione synthesis rates. Thus, at any given intake of cysteine, an increase in glycine intake could be expected to further boost glutathione synthesis and vice versa. Hence, joint supplementation with NAC and glycine could be expected to collaborate in stimulating glutathione production. In the previously cited clinical study,9 the elderly subjects received 100 mg/kg of both NAC and glycine for 14 days, and their red blood cell total glutathione levels (reduced glutathione plus diglutathione) rose from 1.26 mmol/L at baseline to 2.23 mmol/L, a concentration slightly higher than that measured in young subjects who did not take supplements.9 Moreover, the ratio of reduced glutathione to diglutathione rose from 7.4:1 to 16.1:1, indicative of a substantial improvement in redox status as confirmed by concurrent reductions in plasma markers of oxidative stress. The extent to which glycine contributed to the rise in cellular glutathione is unclear because the elderly subjects did not receive glycine or cysteine alone.
Tissue glutathione levels tend to decline with age, a phenomenon that may reflect declining function of Nrf2-mediated induction of phase 2 enzymes (including glutamate cysteine ligase), as well as lower tissue levels of cysteine and glycine.9,18-20 Phase 2 inducer nutraceuticals such as lipoic acid can be used to compensate for this decline in Nrf2 activity.18 An epidemiologic analysis found that while relatively low protein intakes predicted lower subsequent mortality in subjects <65 years, the opposite pattern was seen in those >65 years; greater mortality was seen in those in whom protein accounted for a lower percentage of total calories.21 Suboptimal cysteine intakes, exacerbating the age-related decline in glutathione levels, could play a mediating role in this phenomenon.4,22 An increase in glycine intake could potentially likewise have a beneficial impact on mortality rates in the elderly, most notably in those with low total protein intake.
HEALTH-PROTECTIVE POTENTIAL OF SUPPLEMENTAL GLYCINE
In addition to its role as a glutathione precursor, glycine is a substrate in the synthesis of porphyrins (heme), purines, creatine, sarcosine, and bile salts.23 Glycine constitutes approximately one-third of the amino acids in collagen and elastin and, hence, is a prominent component of connective tissues and the extracellular matrix. Additionally, glycine is an agonist for glycine-gated chloride channels that are expressed by a range of tissues peripherally and in the central nervous system.24-28 Activation of glycine-gated chloride channels has a depolarizing impact on cells that actively accumulate chloride and a hyperpolarizing impact on those that do not. Plasma glycine levels are close to the Km for glycine-gated chloride channels, so supplemental glycine, which can raise plasma glycine levels severalfold, tends to enhance the activity of these channels.29,30 Glycine is also a coagonist for certain neuronal N-methyl-D-aspartate (NMDA) channels.31,32 Additionally, glycine can suppress protein glycation and subsequent formation of advanced glycation end products by competing with protein-bound lysines for formation of Schiff bases with reactive aldehydes.33,34 In hepatocytes almost exclusively, glycine is metabolized to yield pyruvate (2 glycines generate 1 pyruvate); pyruvate can function as a scavenging antioxidant as it quenches hydrogen peroxide.35-39
GLYCINE AND METABOLIC SYNDROME
Owing to these diverse activities, supplemental glycine has shown intriguing and often beneficial effects in preclinical and clinical studies. Glycine provides protection from the adverse metabolic impacts of a high-fructose diet in rats, exerting favorable effects on insulin sensitivity, blood pressure, serum free fatty acids, and intraabdominal fat stores.40 Conceivably, this phenomenon reflects in part an upregulation of enteral production of glucagon-like peptide-1 (GLP-1).41 The L-cells that secrete GLP-1 express glycine-gated chloride channels; because L-cells accumulate chloride, activation of these channels exerts a depolarizing effect that promotes calcium influx and GLP-1 secretion.28 Although the impact of supplemental glycine on GLP-1 production has not been assessed in vivo, oral gelatin, notably rich in glycine, has been shown to boost GLP-1 levels.42,43 Glycine administered in a morning fasting metabolism has been shown to provoke increased glucagon secretion, likely reflecting a direct or indirect stimulatory effect on pancreatic alpha cells.44 Because both GLP-1 and glucagon boost hepatic fatty acid oxidation, these hormones might collaborate in mediating the protective impact of supplemental glycine on fructose-fed rats.41
Díaz-Flores et al administered 15-g glycine daily (5 g, 3 times daily) to patients with metabolic syndrome. Despite fasting glucose rising significantly from 101 mg/dL to 114 mg/dL (P=0.001), glycated hemoglobin fell from 7.81% to 6.45% (P=0.0001); the increase in fasting glucose in these patients might be explained by a stimulatory effect of glycine on glucagon secretion.45 Glycine supplementation was also associated with significant reductions in systolic blood pressure and plasma markers of oxidative stress.45 Importantly, when 5-g glycine was administered along with 25-g glucose in an oral glucose tolerance test, the subsequent increase in plasma glucose was notably blunted compared to the response to glucose alone. Because the glycine had relatively little impact on insulin secretion, the authors hypothesized that glycine was promoting intestinal production of an unknown hormone capable of boosting insulin sensitivity.44 When patients with type 2 diabetes were administered 5-g glycine 4 times daily for 6 months in an uncontrolled clinical trial, glycated hemoglobin fell from 9.6% to 6.9% (P<0.05); a favorable impact of glycine on oral glucose tolerance and on protein glycation might have collaborated in producing this benefit.46 A reduction of glycated hemoglobin in glycine-treated diabetic rats has also been reported.47 In rats with streptozotocin-induced diabetes, a favorable effect on certain diabetes complications (ie, glomerulosclerosis, cataracts, and microaneurysms of the retinal arteries) has been found.48-50
ANTIINFLAMMATORY EFFECTS OF GLYCINE
Macrophages, leukocytes, and Kupffer cells express glycine-gated chloride channels, and in these cells glycine exerts a hyperpolarizing effect, inhibiting calcium influx via voltage-sensitive calcium channels and thereby downregulating their proinflammatory activity.25,51,52 Studies have shown that dietary glycine can protect the livers of alcohol-fed rodents, and this effect is thought to reflect, in part, a suppression of Kupffer cell activation that lessens production of tumor necrosis factor alpha.53-56 Likewise, glycine administration improves liver status in rat models of nonalcoholic steatohepatitis induced by a high-fat, high-sugar diet or by methionine/choline deficiency.57-59 Increased hepatic production of pyruvate might contribute to the protection observed in these studies. Glycine supplementation is also beneficial in rodent models of inflammatory arthritis,60,61 although it had little impact on a canine model of osteoarthritis.62
GLYCINE AND VASCULAR HEALTH
Endothelial cells, and likely foam cells, express glycine-gated chloride channels.24 Glycine can exert a hyperpolarizing effect on endothelial cells that would enhance calcium influx.30,63 Endothelial cells lack voltage-sensitive calcium channels, so hyperpolarization therefore tends to drive calcium influx. Endothelial calcium influx activates nitric oxide synthase and also boosts expression of this enzyme via a mechanism dependent on a calcium/calmodulin-dependent kinase.64,65 Additionally, whereas membrane depolarization increases endothelial superoxide production via nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activation, hyperpolarization has the opposite effect.66-68 Hence, supplemental glycine, via its hyperpolarizing effect on vascular endothelium and also via an antiinflammatory impact on foam cells, has been proposed to exert an antiatherosclerotic effect, a proposition that has not yet been tested.30 Consistent with this possibility, glycine administration to aging rats in drinking water for 2 months amplified endothelium-dependent vasodilation, increased mRNA expression of endothelial nitric oxide synthase, and downregulated expression of cyclooxygenase-2 and tumor necrosis factor alpha.69
Platelets likewise express glycine-responsive chloride channels; these channels have a hyperpolarizing action that suppresses calcium influx and promotes platelet stability.26
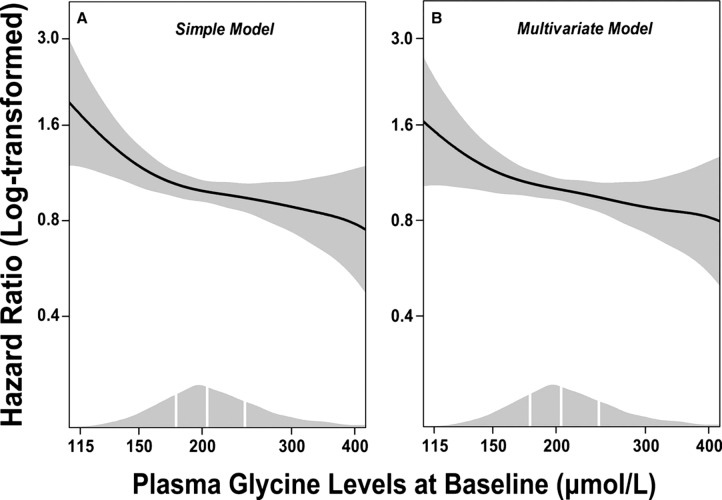
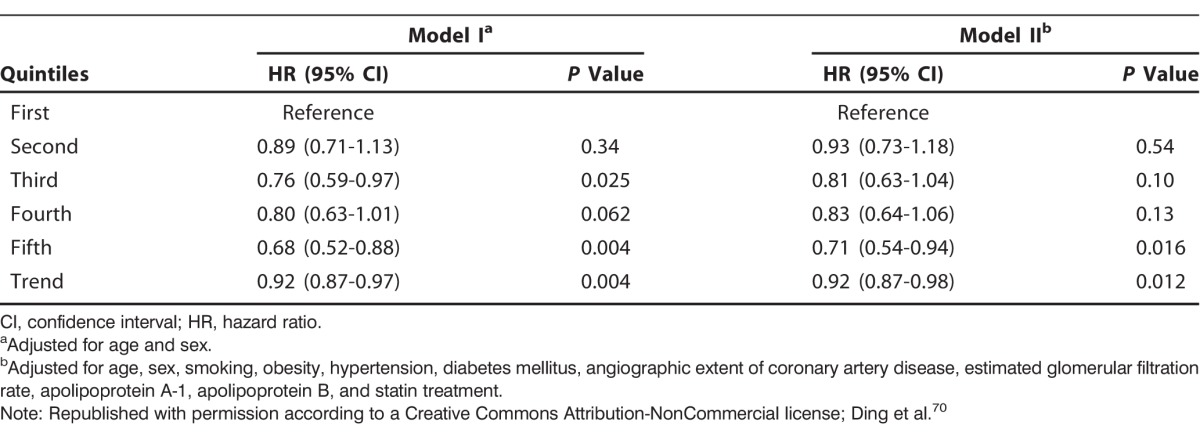
Ding et al measured plasma glycine levels in >4,000 patients who received coronary angiography for suspected angina pectoris.70 Higher levels of glycine were associated with a lesser extent of coronary atheroma and lower prevalence of obesity, hypertension, and diabetes. In light of the potential antiinflammatory effects of glycine, it is notable that C-reactive protein levels were nearly twice as high in patients in the bottom quartile of plasma glycine as in the upper quartile (2.48 vs 1.35 mg/L, P<0.001). During a median follow-up of 7.4 years, 616 patients experienced myocardial infarcts. After multivariate adjustments for known risk factors, patients whose glycine was in the fifth quintile at baseline were 29% less likely to have experienced an infarct than patients in the first quintile (hazard ratio 0.71; 95% confidence interval 0.54-0.94, P=0.016) (Figure and Table). Whether these associations reflect cause-effect relationships remains to be determined, but the findings are consistent with the possibility that elevated glycine levels provide protection from cardiovascular events. Of related interest is a report that dietary glycine prevents development of cardiac hypertrophy in rats subjected to pressure overload.71 This effect may be mediated by glycine receptors expressed on cardiomyocytes.27,71
Figure.
Dose-response associations between (log-transformed) plasma glycine and risk of acute myocardial infarction. Generalized additive regressions are used with the adjustment for age and sex in the simple model (A) and with additional adjustment for smoking, obesity, hypertension, diabetes mellitus, angiographic extent of coronary artery disease (ordinal), estimated glomerular filtration rate, apolipoprotein A-1, apolipoprotein B, and statin treatment in the multivariate model (B). The solid lines and the shaded areas represent hazard ratios of plasma glycine and their 95% confidence intervals, respectively. The areas under the curve along the x-axes represent the distributions of the plasma glycine concentrations (μmol/L) in the total population. The vertical white lines denote the 25th, 50th, and 75th percentiles of plasma glycine, respectively. (Republished with permission according to a Creative Commons Attribution-NonCommercial license; Ding et al.70)
Table.
Hazard Ratios of Acute Myocardial Infarction According to Quintiles of Plasma Glycine Levels
IMPACTS ON CANCER, CONNECTIVE TISSUE, AND THE CENTRAL NERVOUS SYSTEM
Glycine might prove to have some utility in cancer management. Indeed, glycine supplementation has been reported to slow the growth of various implanted tumors in rodents, likely owing to an antiangiogenic impact on endothelial cells.72-74 However, this effect might modestly retard wound healing.73 The antiangiogenic effect of glycine may reflect the fact that activation of endothelial NADPH oxidase, which the hyperpolarizing action of glycine may oppose, is a key mediator of the signaling pathway whereby vascular endothelial growth factor and its Flk-1 receptor promote proliferation and migration of endothelial cells.75,76 Additionally, glycine acts directly on a human hepatocyte line, via glycine receptors, to inhibit expression of vascular endothelial growth factor; proliferation of these cells was not influenced by glycine.77 The antiinflammatory effects of glycine may extend to cancer cachexia, as glycine administration has been found to blunt the loss of muscle and fat mass and decrease inflammatory markers in mice implanted with C26 cancer cells.78
The possibility that increased glycine intake may have a favorable impact on bones and tendons is being evaluated. In vitro, glycine was found to stimulate the proliferation of an osteoblast cell line; when administered to ovariectomized rats, glycine favorably influenced bone mineral density and ultrastructure.79 In a clinical study evaluating monozygotic twins, the twins with a higher dietary intake of glycine or alanine tended to have higher spinal bone mineral density.80 In a rat model of Achilles tendinitis, supplemental glycine aided healing.81-83 The fact that collagen and elastin are remarkably rich in glycine suggests that optimal glycine nutrition might be beneficial for the health of connective tissues (including the cardiovascular system).23
Finally, glycine, via its interaction with glycine and NMDA receptors, has potential to influence the central nervous system.
A controlled clinical trial found that glycine supplementation prior to bedtime improves sleep quality in patients afflicted with insomnia.84 However, daytime administration of glycine did not cause drowsiness.85 In rats, glycine administration boosts non–rapid eye movement sleep and amplifies the decline in body temperature (reflecting cutaneous vasodilation) that typically accompanies sleep. These effects were found to be mediated by NMDA receptors in the suprachiasmatic nucleus.86 Glycine has also been evaluated as an adjuvant to neuroleptic treatment of schizophrenia; both high-dose glycine and D-serine, a full agonist for the glycine-binding site on NMDA receptors, have been reported to aid control of negative symptoms.87,88
GLYCINE AS A SUPPLEMENTAL NUTRIENT
Clinical studies of supplemental glycine that have achieved interesting results have used daily glycine doses ranging from 3 g to as high as 0.8 g/kg, split into 2-4 servings.45,46,85,89 These studies have failed to note any significant side effects, presumably reflecting the fact that glycine is efficiently absorbed and hence does not cause diarrhea.45,46,85,89 Ample doses of glycine are not impractical because glycine is inexpensive, has a mildly sweet flavor, is highly and rapidly soluble, and can be added to a wide range of beverages.
CONCLUSION
We have good reason to suspect that clinically feasible doses of oral glycine can boost tissue levels of glutathione, an effect that could be amplified by concurrent administration of NAC and/or a phase 2 inducer such as lipoic acid. Because glutathione levels decline during the aging process, supplemental glycine would likely have its most notable impact in elderly people with diets relatively low in protein. Moreover, by serving as a precursor for a range of biomolecules, by acting as an agonist for glycine-gated chloride channels and a coagonist for NMDA receptors, and by suppressing protein glycation, increased intakes of glycine have the potential to exert a broad range of protective effects: inhibiting atherosclerosis and cardiac hypertrophy, controlling metabolic syndrome, aiding control of diabetes and its complications, protecting the liver exposed to excess alcohol or fatty acids, blunting inflammation, and aiding sleep quality. Clinical research is warranted to evaluate the impact of supplemental glycine on glutathione levels and on various health disorders.
ACKNOWLEDGMENTS
Mark F. McCarty is coowner and science director of a nutraceutical company that sells a product containing glycine. James H. O'Keefe is the chief medical officer and founder of CardioTabs, a nutraceutical company. James J. DiNicolantonio has no conflicts of interest to disclose.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care and Medical Knowledge.
REFERENCES
- 1. Dickinson DA., Forman HJ. Glutathione in defense and signaling: lessons from a small thiol. Ann N Y Acad Sci. 2002. November; 973: 488- 504. [DOI] [PubMed] [Google Scholar]
- 2. Shelton MD., Chock PB., Mieyal JJ. Glutaredoxin: role in reversible protein s-glutathionylation and regulation of redox signal transduction and protein translocation. Antioxid Redox Signal. 2005. Mar-Apr; 7 3-4: 348- 366. [DOI] [PubMed] [Google Scholar]
- 3. Parsons ZD., Gates KS. Thiol-dependent recovery of catalytic activity from oxidized protein tyrosine phosphatases. Biochemistry. 2013. September 17; 52 37: 6412- 6423. 10.1021/bi400451m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dröge W., Kinscherf R., Hildebrandt W., Schmitt T. The deficit in low molecular weight thiols as a target for antiageing therapy. Curr Drug Targets. 2006. November; 7 11: 1505- 1512. [DOI] [PubMed] [Google Scholar]
- 5. Atkuri KR., Mantovani JJ., Herzenberg LA., Herzenberg LA. N-Acetylcysteine—a safe antidote for cysteine/glutathione deficiency. Curr Opin Pharmacol. 2007. August; 7 4: 355- 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dodd S., Dean O., Copolov DL., Malhi GS., Berk M. N-acetylcysteine for antioxidant therapy: pharmacology and clinical utility. Expert Opin Biol Ther. 2008. December; 8 12: 1955- 1962. 10.1517/14728220802517901. [DOI] [PubMed] [Google Scholar]
- 7. Brown TR., Drummond ML., Barelier S., et al. Aspartate 458 of human glutathione synthetase is important for cooperativity and active site structure. Biochem Biophys Res Commun. 2011. August 5; 411 3: 536- 542. 10.1016/j.bbrc.2011.06.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Njålsson R., Carlsson K., Olin B., et al. Kinetic properties of missense mutations in patients with glutathione synthetase deficiency. Biochem J. 2000. July 1; 349 Pt 1: 275- 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sekhar RV., Patel SG., Guthikonda AP., et al. Deficient synthesis of glutathione underlies oxidative stress in aging and can be corrected by dietary cysteine and glycine supplementation. Am J Clin Nutr. 2011. September; 94 3: 847- 853. 10.3945/ajcn.110.003483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Richman PG., Meister A. Regulation of gamma-glutamyl-cysteine synthetase by nonallosteric feedback inhibition by glutathione. J Biol Chem. 1975. February 25; 250 4: 1422- 1426. [PubMed] [Google Scholar]
- 11. Wang W., Dai Z., Wu Z., et al. Glycine is a nutritionally essential amino acid for maximal growth of milk-fed young pigs. Amino Acids. 2014. August; 46 8: 2037- 2045. 10.1007/s00726-014-1758-3. [DOI] [PubMed] [Google Scholar]
- 12. Zhang Y., Lv SJ., Yan H., Wang L., Liang GP., Wan QX., Peng X. Effects of glycine supplementation on myocardial damage and cardiac function after severe burn. Burns. 2013. June; 39 4: 729- 735. 10.1016/j.burns.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 13. Senthilkumar R., Viswanathan P., Nalini N. Effect of glycine on oxidative stress in rats with alcohol induced liver injury. Pharmazie. 2004. January; 59 1: 55- 60. [PubMed] [Google Scholar]
- 14. Ruiz-Ramírez A., Ortiz-Balderas E., Cardozo-Saldaña G., Diaz-Diaz E., El-Hafidi M. Glycine restores glutathione and protects against oxidative stress in vascular tissue from sucrose-fed rats. Clin Sci (Lond). 2014. January 1; 126 1: 19- 29. 10.1042/CS20130164. [DOI] [PubMed] [Google Scholar]
- 15. Jackson AA., Badaloo AV., Forrester T., Hibbert JM., Persaud C. Urinary excretion of 5-oxoproline (pyroglutamic aciduria) as an index of glycine insufficiency in normal man. Br J Nutr. 1987. September; 58 2: 207- 214. [DOI] [PubMed] [Google Scholar]
- 16. Persaud C., Jackson AA. 5-L-oxoprolinuria and glycine sufficiency. Clin Chem. 1991. September; 37 9: 1660- 1661. [PubMed] [Google Scholar]
- 17. Jackson AA., Persaud C., Meakins TS., Bundy R. Urinary excretion of 5-L-oxoproline (pyroglutamic acid) is increased in normal adults consuming vegetarian or low protein diets. J Nutr. 1996. November; 126 11: 2813- 2822. [DOI] [PubMed] [Google Scholar]
- 18. Suh JH., Shenvi SV., Dixon BM., et al. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci U S A. 2004. March 9; 101 10: 3381- 3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ungvari Z., Bailey-Downs L., Sosnowska D., et al. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of NRF2-mediated antioxidant response. Am J Physiol Heart Circ Physiol. 2011. August; 301 2: H363- H372. 10.1152/ajpheart.01134.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shih PH., Yen GC. Differential expressions of antioxidant status in aging rats: the role of transcriptional factor Nrf2 and MAPK signaling pathway. Biogerontology. 2007. April; 8 2: 71- 80. [DOI] [PubMed] [Google Scholar]
- 21. Levine ME., Suarez JA., Brandhorst S., et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014. March 4; 19 3: 407- 417. 10.1016/j.cmet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McCarty MF., DiNicolantonio JJ. An increased need for dietary cysteine in support of glutathione synthesis may underlie the increased risk for mortality associated with low protein intake in the elderly. Age (Dordr). 2015. October; 37 5: 96 10.1007/s11357-015-9823-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Meléndez-Hevia E., De Paz-Lugo P., Cornish-Bowden A., Cárdenas ML. A weak link in metabolism: the metabolic capacity for glycine biosynthesis does not satisfy the need for collagen synthesis. J Biosci. 2009. December; 34 6: 853- 872. [DOI] [PubMed] [Google Scholar]
- 24. Yamashina S., Konno A., Wheeler MD., et al. Endothelial cells contain a glycine-gated chloride channel. Nutr Cancer. 2001; 40 2: 197- 204. [DOI] [PubMed] [Google Scholar]
- 25. Froh M., Thurman RG., Wheeler MD. Molecular evidence for a glycine-gated chloride channel in macrophages and leukocytes. Am J Physiol Gastrointest Liver Physiol. 2002. October; 283 4: G856- G863. [DOI] [PubMed] [Google Scholar]
- 26. Schemmer P., Zhong Z., Galli U., et al. Glycine reduces platelet aggregation. Amino Acids. 2013. March; 44 3: 925- 931. 10.1007/s00726-012-1422-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang HD., Lü XX., Lu DX., Qi RB., Wang YP., Fu YM., Wang LW. Glycine inhibits the LPS-induced increase in cytosolic Ca2+ concentration and TNFalpha production in cardiomyocytes by activating a glycine receptor. Acta Pharmacol Sin. 2009. August; 30 8: 1107- 1114. 10.1038/aps.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gameiro A., Reimann F., Habib AM., et al. The neurotransmitters glycine and GABA stimulate glucagon-like peptide-1 release from the GLUTag cell line. J Physiol. 2005. December 15; 569 Pt 3: 761- 772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lynch JW. Molecular structure and function of the glycine receptor chloride channel. Physiol Rev. 2004. October; 84 4: 1051- 1095. [DOI] [PubMed] [Google Scholar]
- 30. McCarty MF., Barroso-Aranda J., Contreras F. The hyperpolarizing impact of glycine on endothelial cells may be anti-atherogenic. Med Hypotheses. 2009. August; 73 2: 263- 264. 10.1016/j.mehy.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 31. Mayer ML., Vyklicky L, Jr, Clements J. Regulation of NMDA receptor desensitization in mouse hippocampal neurons by glycine. Nature. 1989. March 30; 338 6214: 425- 427. [DOI] [PubMed] [Google Scholar]
- 32. Hirai H., Kirsch J., Laube B., Betz H., Kuhse J. The glycine binding site of the N-methyl-D-aspartate receptor subunit NR1: identification of novel determinants of co-agonist potentiation in the extracellular M3-M4 loop region. Proc Natl Acad Sci U S A. 1996. June 11; 93 12: 6031- 6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ramakrishnan S., Sulochana KN. Decrease in glycation of lens proteins by lysine and glycine by scavenging of glucose and possible mitigation of cataractogenesis. Exp Eye Res. 1993. November; 57 5: 623- 628. [DOI] [PubMed] [Google Scholar]
- 34. Ramakrishnan S., Sulochana KN., Punitham R. Free lysine, glycine, alanine, glutamic acid and aspartic acid reduce the glycation of human lens proteins by galactose. Indian J Biochem Biophys. 1997. December; 34 6: 518- 523. [PubMed] [Google Scholar]
- 35. Xue HH., Fujie M., Sakaguchi T., et al. Flux of the L-serine metabolism in rat liver. The predominant contribution of serine dehydratase. J Biol Chem. 1999. June 4; 274 23: 16020- 16027. [DOI] [PubMed] [Google Scholar]
- 36. Lee HH., Kim DJ., Ahn HJ., Ha JY., Suh SW. Crystal structure of T-protein of the glycine cleavage system: cofactor binding, insights into H-protein recognition, and molecular basis for understanding nonketotic hyperglycinemia. J Biol Chem. 2004. November 26; 279 48: 50514- 50523. [DOI] [PubMed] [Google Scholar]
- 37. Szebenyi DM., Musayev FN., di Salvo ML., Safo MK., Schirch V. Serine hydroxymethyltransferase: role of glu75 and evidence that serine is cleaved by a retroaldol mechanism. Biochemistry. 2004. June 8; 43 22: 6865- 6876. [DOI] [PubMed] [Google Scholar]
- 38. Ogawa H., Fujioka M., Su Y., Kanamoto R., Pitot HC. Nutritional regulation and tissue-specific expression of the serine dehydratase gene in rat. J Biol Chem. 1991. October 25; 266 30: 20412- 20417. [PubMed] [Google Scholar]
- 39. Lopalco A., Stella VJ. Effect of molecular structure on the relative hydrogen peroxide scavenging ability of some α-keto carboxylic acids. J Pharm Sci. 2016. September; 105 9: 2879- 2885. 10.1016/j.xphs.2016.03.041. [DOI] [PubMed] [Google Scholar]
- 40. El Hafidi M., Pérez I., Zamora J., Soto V., Carvajal-Sandoval G., Baños G. Glycine intake decreases plasma free fatty acids, adipose cell size, and blood pressure in sucrose-fed rats. Am J Physiol Regul Integr Comp Physiol. 2004. December; 287 6: R1387- R1393. [DOI] [PubMed] [Google Scholar]
- 41. McCarty MF., DiNicolantonio JJ. The cardiometabolic benefits of glycine: is glycine an ‘antidote' to dietary fructose? Open Heart. 2014. May 28; 1 1: e000103 10.1136/openhrt-2014-000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Karamanlis A., Chaikomin R., Doran S., et al. Effects of protein on glycemic and incretin responses and gastric emptying after oral glucose in healthy subjects. Am J Clin Nutr. 2007. November; 86 5: 1364- 1368. [DOI] [PubMed] [Google Scholar]
- 43. Rubio IG., Castro G., Zanini AC., Medeiros-Neto G. Oral ingestion of a hydrolyzed gelatin meal in subjects with normal weight and in obese patients: postprandial effect on circulating gut peptides, glucose and insulin. Eat Weight Disord. 2008. March; 13 1: 48- 53. [DOI] [PubMed] [Google Scholar]
- 44. Gannon MC., Nuttall JA., Nuttall FQ. The metabolic response to ingested glycine. Am J Clin Nutr. 2002. December; 76 6: 1302- 1307. [DOI] [PubMed] [Google Scholar]
- 45. Díaz-Flores M., Cruz M., Duran-Reyes G., et al. Oral supplementation with glycine reduces oxidative stress in patients with metabolic syndrome, improving their systolic blood pressure. Can J Physiol Pharmacol. 2013. October; 91 10: 855- 860. 10.1139/cjpp-2012-0341. [DOI] [PubMed] [Google Scholar]
- 46. Carvajal Sandoval G, Medina Santillán R, Juárez E, Ramos Martínez G, Carvajal Juárez ME. Effect of glycine on hemoglobin glycation in diabetic patients. Proc West Pharmacol Soc. 1999; 42: 31- 32. [PubMed] [Google Scholar]
- 47. Carvajal Sandoval G, Juárez E, Ramos Martínez G, Carvajal Juárez ME, Medina-Santillán R. Inhibition of hemoglobin glycation with glycine in induced diabetes mellitus in rats. Proc West Pharmacol Soc. 1999; 42: 35- 36. [PubMed] [Google Scholar]
- 48. Alvarado-Vásquez N., Zamudio P., Cerón E., Vanda B., Zenteno E., Carvajal-Sandoval G. Effect of glycine in streptozotocin-induced diabetic rats. Comp Biochem Physiol C Toxicol Pharmacol. 2003. April; 134 4: 521- 527. [DOI] [PubMed] [Google Scholar]
- 49. Alvarado-Vásquez N., Lascurain R., et al. Oral glycine administration attenuates diabetic complications in streptozotocin-induced diabetic rats. Life Sci. 2006. June 13; 79 3: 225- 232. [DOI] [PubMed] [Google Scholar]
- 50. Bahmani F., Bathaie SZ., Aldavood SJ., Ghahghaei A. Glycine therapy inhibits the progression of cataract in streptozotocin-induced diabetic rats. Mol Vis. 2012; 18: 439- 448. [PMC free article] [PubMed] [Google Scholar]
- 51. Wheeler M., Stachlewitz RF., Yamashina S., Ikejima K., Morrow AL., Thurman RG. Glycine-gated chloride channels in neutrophils attenuate calcium influx and superoxide production. FASEB J. 2000. March; 14 3: 476- 484. [DOI] [PubMed] [Google Scholar]
- 52. Wheeler MD., Thurman RG. Production of superoxide and TNF-alpha from alveolar macrophages is blunted by glycine. Am J Physiol. 1999. November; 277 5 Pt 1: L952- L959. [DOI] [PubMed] [Google Scholar]
- 53. Yin M., Ikejima K., Arteel GE., et al. Glycine accelerates recovery from alcohol-induced liver injury. J Pharmacol Exp Ther. 1998. August; 286 2: 1014- 1019. [PubMed] [Google Scholar]
- 54. Wheeler MD., Ikejema K., Enomoto N., et al. Glycine: a new anti-inflammatory immunonutrient. Cell Mol Life Sci. 1999. November 30; 56 9-10: 843- 856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yamashina S., Ikejima K., Enomoto N., Takei Y., Sato N. Glycine as a therapeutic immuno-nutrient for alcoholic liver disease. Alcohol Clin Exp Res. 2005. November; 29 11 Suppl: 162S- 165S. [DOI] [PubMed] [Google Scholar]
- 56. Bruns H., Watanpour I., Gebhard MM., et al. Glycine and taurine equally prevent fatty livers from Kupffer cell-dependent injury: an in vivo microscopy study. Microcirculation. 2011. April; 18 3: 205- 213. 10.1111/j.1549-8719.2010.00078.x. [DOI] [PubMed] [Google Scholar]
- 57. Zhou X., Han D., Xu R., et al. Glycine protects against high sucrose and high fat-induced non-alcoholic steatohepatitis in rats. Oncotarget. 2016. December 6; 7 49: 80223- 80237. 10.18632/oncotarget.12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Barakat HA., Hamza AH. Glycine alleviates liver injury induced by deficiency in methionine and or choline in rats. Eur Rev Med Pharmacol Sci. 2012. June; 16 6: 728- 736. [PubMed] [Google Scholar]
- 59. Dou ZF., Guo YR., Liu JC., et al. Ameliorative effects of glycine in an experimental nonalcoholic steatohepatitis and its correlation between TREM-1 and TREM-2. Am J Transl Res. 2016. February 15; 8 2: 284- 297. [PMC free article] [PubMed] [Google Scholar]
- 60. Li X., Bradford BU., Wheeler MD., et al. Dietary glycine prevents peptidoglycan polysaccharide-induced reactive arthritis in the rat: role for glycine-gated chloride channel. Infect Immun. 2001. September; 69 9: 5883- 5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hartog A., Leenders I., van der Kraan PM., Garssen J. Anti-inflammatory effects of orally ingested lactoferrin and glycine in different zymosan-induced inflammation models: evidence for synergistic activity. Int Immunopharmacol. 2007. December 15; 7 13: 1784- 1792. [DOI] [PubMed] [Google Scholar]
- 62. Mastbergen SC., Frost-Christensen LN., Hartog A., DeGroot J., Hazewinkel HA., Lafeber FP. Oral glycine in treatment of canine experimental osteoarthritis. Osteoarthritis and Cartilage. 2007. December; 15: C224- C225. [Google Scholar]
- 63. Lückhoff A., Busse R. Activators of potassium channels enhance calcium influx into endothelial cells as a consequence of potassium currents. Naunyn Schmiedebergs Arch Pharmacol. 1990. July; 342 1: 94- 99. [DOI] [PubMed] [Google Scholar]
- 64. Wang W., Ha CH., Jhun BS., Wong C., Jain MK., Jin ZG. Fluid shear stress stimulates phosphorylation-dependent nuclear export of HDAC5 and mediates expression of KLF2 and eNOS. Blood. 2010. April 8; 115 14: 2971- 2979. 10.1182/blood-2009-05-224824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Liu G., Han J., Profirovic J., Strekalova E., Voyno-Yasenetskaya TA. Galpha13 regulates MEF2-dependent gene transcription in endothelial cells: role in angiogenesis. Angiogenesis. 2009; 12 1: 1- 15. 10.1007/s10456-008-9123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sohn HY., Keller M., Gloe T., Morawietz H., Rueckschloss U., Pohl U. The small G-protein Rac mediates depolarization-induced superoxide formation in human endothelial cells. J Biol Chem. 2000. June 23; 275 25: 18745- 18750. [DOI] [PubMed] [Google Scholar]
- 67. Zhang Q., Matsuzaki I., Chatterjee S., Fisher AB. Activation of endothelial NADPH oxidase during normoxic lung ischemia is KATP channel dependent. Am J Physiol Lung Cell Mol Physiol. 2005. December; 289 6: L954- L961. [DOI] [PubMed] [Google Scholar]
- 68. McCabe RD., Bakarich MA., Srivastava K., Young DB. Potassium inhibits free radical formation. Hypertension. 1994. July; 24 1: 77- 82. [DOI] [PubMed] [Google Scholar]
- 69. Gómez-Zamudio JH., García-Macedo R., Lázaro-Suárez M., Ibarra-Barajas M., Kumate J., Cruz M. Vascular endothelial function is improved by oral glycine treatment in aged rats. Can J Physiol Pharmacol. 2015. June; 93 6: 465- 473. 10.1139/cjpp-2014-0393. [DOI] [PubMed] [Google Scholar]
- 70. Ding Y., Svingen GF., Pedersen ER., et al. Plasma glycine and risk of acute myocardial infarction in patients with suspected stable angina pectoris. J Am Heart Assoc. 2015. December 31; 5 1 10.1161/JAHA.115.002621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lu Y., Zhu X., Li J., et al. Glycine prevents pressure overload induced cardiac hypertrophy mediated by glycine receptor. Biochem Pharmacol. 2017. January 1; 123: 40- 51. 10.1016/j.bcp.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 72. Rose ML., Madren J., Bunzendahl H., Thurman RG. Dietary glycine inhibits the growth of B16 melanoma tumors in mice. Carcinogenesis. 1999. May; 20 5: 793- 798. [DOI] [PubMed] [Google Scholar]
- 73. Amin K., Li J., Chao WR., Dewhirst MW., Haroon ZA. Dietary glycine inhibits angiogenesis during wound healing and tumor growth. Cancer Biol Ther. 2003. Mar-Apr; 2 2: 173- 178. [DOI] [PubMed] [Google Scholar]
- 74. Bruns H., Kazanavicius D., Schultze D., et al. Glycine inhibits angiogenesis in colorectal cancer: role of endothelial cells. Amino Acids. 2016. November; 48 11: 2549- 2558. [DOI] [PubMed] [Google Scholar]
- 75. Abid MR., Kachra Z., Spokes KC., Aird WC. NADPH oxidase activity is required for endothelial cell proliferation and migration. FEBS Lett. 2000. December 15; 486 3: 252- 256. [DOI] [PubMed] [Google Scholar]
- 76. Ushio-Fukai M. Redox signaling in angiogenesis: role of NADPH oxidase. Cardiovasc Res. 2006. July 15; 71 2: 226- 235. [DOI] [PubMed] [Google Scholar]
- 77. Bruns H., Petrulionis M., Schultze D., et al. Glycine inhibits angiogenic signaling in human hepatocellular carcinoma cells. Amino Acids. 2014. April; 46 4: 969- 976. 10.1007/s00726-013-1662-2. [DOI] [PubMed] [Google Scholar]
- 78. Ham DJ., Murphy KT., Chee A., Lynch GS., Koopman R. Glycine administration attenuates skeletal muscle wasting in a mouse model of cancer cachexia. Clin Nutr. 2014. June; 33 3: 448- 458. 10.1016/j.clnu.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 79. Kim MH., Kim HM., Jeong HJ. Estrogen-like osteoprotective effects of glycine in in vitro and in vivo models of menopause. Amino Acids. 2016. March; 48 3: 791- 800. 10.1007/s00726-015-2127-6. [DOI] [PubMed] [Google Scholar]
- 80. Jennings A., MacGregor A., Spector T., Cassidy A. Amino acid intakes are associated with bone mineral density and prevalence of low bone mass in women: evidence from discordant monozygotic twins. J Bone Miner Res. 2016. February; 31 2: 326- 335. 10.1002/jbmr.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Vieira CP., De Oliveira LP., Da Ré Guerra F., et al. Glycine improves biochemical and biomechanical properties following inflammation of the achilles tendon. Anat Rec (Hoboken). 2015. March; 298 3: 538- 545. 10.1002/ar.23041. [DOI] [PubMed] [Google Scholar]
- 82. Vieira CP. Guerra Fda R, de Oliveira LP, Almeida MS, Marcondes MC, Pimentell ER. Green tea and glycine aid in the recovery of tendinitis of the Achilles tendon of rats. Connect Tissue Res. 2015. February; 56 1: 50- 58. 10.3109/03008207.2014.983270. [DOI] [PubMed] [Google Scholar]
- 83. Vieira CP., De Oliveira LP. Da Ré Guerra F, Marcondes MC, Pimentel ER. Green tea and glycine modulate the activity of metalloproteinases and collagen in the tendinitis of the myotendinous junction of the Achilles tendon. Anat Rec (Hoboken). 2016. July; 299 7: 918- 928. 10.1002/ar.23361. [DOI] [PubMed] [Google Scholar]
- 84. Bannai M., Kawai N. New therapeutic strategy for amino acid medicine: glycine improves the quality of sleep. J Pharmacol Sci. 2012; 118 2: 145- 148. [DOI] [PubMed] [Google Scholar]
- 85. Bannai M., Kawai N., Ono K., Nakahara K., Murakami N. The effects of glycine on subjective daytime performance in partially sleep-restricted healthy volunteers. Front Neurol. 2012. April 18; 3: 61 10.3389/fneur.2012.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kawai N., Sakai N., Okuro M., et al. The sleep-promoting and hypothermic effects of glycine are mediated by NMDA receptors in the suprachiasmatic nucleus. Neuropsychopharmacology. 2015. May; 40 6: 1405- 1416. 10.1038/npp.2014.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Heresco-Levy U., Silipo G., Javitt DC. Glycinergic augmentation of NMDA receptor-mediated neurotransmission in the treatment of schizophrenia. Psychopharmacol Bull. 1996; 32 4: 731- 740. [PubMed] [Google Scholar]
- 88. Tsai G., Yang P., Chung LC., Lange N., Coyle JT. D-serine added to antipsychotics for the treatment of schizophrenia. Biol Psychiatry. 1998. December 1; 44 11: 1081- 1089. [DOI] [PubMed] [Google Scholar]
- 89. Heresco-Levy U., Javitt DC., Ermilov M., Mordel C., Silipo G., Lichtenstein M. Efficacy of high-dose glycine in the treatment of enduring negative symptoms of schizophrenia. Arch Gen Psychiatry. 1999. January; 56 1: 29- 36. [DOI] [PubMed] [Google Scholar]