Abstract
Amyotrophic lateral sclerosis (ALS) is a multifactorial disease with limited therapeutic options. Numerous intrinsic and extrinsic factors are involved in ALS motor neuron degeneration. One possible effector accelerating motor neuron death in ALS is damage to the blood-Central Nervous System barrier (B-CNS-B), mainly due to endothelial cell (EC) degeneration. Although mechanisms of EC damage in ALS are still unknown, vascular impairment may be initiated by various humoral inflammatory factors and other mediators. Systemic IL-6-mediated inflammation is a possible early extrinsic effector leading to the EC death causing central nervous system (CNS) barrier damage. In this review, we discuss the potential role of humoral factors in triggering EC alterations in ALS. A specific focus was on humoral IL-6 cytokine mediating EC inflammation via the trans-signaling pathway. Our preliminary in vitro studies demonstrated a proof of principle that short term exposure of human bone marrow endothelial cells to plasma from ALS patient leads to cell morphological changes, significantly upregulated IL-6R immunoexpression, and pro-inflammatory cell response. Our in-depth understanding of specific molecular mechanisms of this humoral cytokine in EC degeneration may facilitate an endothelial-IL-6-targeting therapy for restoring cell homeostasis and eventually reestablishing B-CNS-B integrity in ALS.
Keywords: ALS, IL-6 cytokine, endothelial cells, inflammation
1. Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease affecting motor neurons in the brain and spinal cord. Progression of this disease leads to paralysis and death of the patient, usually within five years of diagnosis [1,2,3]. About 90–95% of ALS cases are sporadic (SALS) while the remaining cases are genetically linked or familial (FALS). Men have a higher incidence of ALS than women and peak ages for initial disease symptoms are 58–63 years for SALS and 47–52 years for FALS [4]. In FALS cases, various mutations in genes coding for Cu/Zn superoxide dismutase 1 (SOD1), TARDBP (TDP-43), FUS/TLS, ANG, and C90RF72 have been identified (reviewed in [5,6,7,8,9]). Although a mutation in the C90RF72 gene was mainly associated with FALS, this gene mutation has also been found in some SALS cases [10,11]. The clinical presentation and underlying pathology of SALS and FALS are similar. Initially, muscle weakness and twitching or cramping of legs or arms appear in ALS patients. As the disease progresses, muscle atrophy, loss of motor control, and decreased range or stamina are observed. Also, dysarthria, dysphagia, fasciculations, and hyperreflexia are common features of ALS, depending upon the upper and/or lower motor neuron dysfunction. At the end disease stage, muscular paralysis and death occur due to respiratory failure. These clinical disease manifestations have been discussed in detail (reviewed in [12,13,14,15,16,17]). However, regardless of the part of the body first affected by the disease, muscle weakness and atrophy spread to other parts of the body as the disease progresses. Developing specific tools for evaluation of clinical symptoms in ALS patients is very important not only for early diagnosis but also for measuring disease progression, i.e., monitoring swallowing or dysphagia [18,19]. In spite of intensive research on ALS pathogenesis, numerous intrinsic and extrinsic factors in motor neuron death (reviewed in [15,20,21,22,23,24]) limit therapeutic options. The only USA Food and Drug Administration approved drugs for ALS are riluzole [25] and the recently approved edaravone (Radicava®, Mitsubishi Tanabe Pharma Corporation, Osaka, Japan) [26]. Riluzole acts to block the release of excitotoxic glutamate [27] while edaravone has anti-oxidant properties [26].
One possible effector accelerating motor neuron death in ALS is damage to the blood-CNS barrier [28], which separates the CNS tissue from detrimental factors in the systemic circulation. Impairment of the blood-brain barrier (BBB) and blood-spinal cord barrier (BSCB), (collectively, the blood-CNS barrier, B-CNS-B), has been shown in a mouse model of disease and in ALS patients [29,30,31,32,33,34,35,36,37,38]. Our [29,30,31,32] and other [33,34,35,36,37,38] studies demonstrated degeneration of microvessel endothelial cells (EC) and perivascular astrocyte end-feet processes, impairment of the endothelial transport system, and dysfunction of tight junction proteins, deficiencies associated with compromised barrier integrity in the brain and spinal cord, which lead to blood vessel leakage in motor neuron areas. Thus, vascular damage may be an early ALS pathological event [33,34,35]. These and other recent discoveries may identify ALS as a neurovascular disease [32,39,40]. However, mechanism(s) of EC degeneration in ALS is still unknown.
Since the CNS endothelium is a specialized barrier isolating the blood compartment from brain/spinal cord parenchyma, initial microvascular EC damage may be due to blood-derived inflammatory and other mediators in ALS. Elevated systemic levels of inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin (IL)-6, IL-8, interferon-beta (IFN-β), and other interleukins have been identified in ALS [41,42,43,44,45]. Moreover, these peripheral biomarkers not only indicate ongoing inflammatory processes in ALS patients, but also may be used to distinguish ALS patients from patients with other neurological diseases [7,46] and even to predict ALS prognosis [47]. Also, increased cytokine levels detected in blood from ALS patients could be important mediators of the peripheral inflammatory response, either by promoting neuroprotection or accelerating disease progression. Notably, IL-8 is not only an inflammatory cytokine with chemoattractive activity predominantly for neutrophils, but is also a potent angiogenic factor [48]. However, our particular interest is mechanisms of IL-6 actions since this bi-functional cytokine can serve as an anti- or pro-inflammatory mediator [49,50,51,52]. Recognizing IL-6′s dual actions, it will be critical to monitor humoral expression levels of this cytokine during disease progression in ALS patients. Peripheral IL-6 upregulation likely corresponds to an inflammatory cell response exacerbating EC damage. This correspondence identifies the IL-6 cytokine as a potential target for a future modulating therapy in ALS.
In this review, we discuss the potential role of humoral factors for triggering EC alterations in ALS. A specific focus was on humoral IL-6 cytokine as a latent early extrinsic inflammatory effector leading to EC damage. Our understanding of specific molecular mechanisms of this cytokine in EC degeneration may foster development of targeted therapies for restoring cell homeostasis and eventually restoring B-CNS-B integrity in ALS.
2. Humoral Effectors in ALS Patients
B-CNS-B impairment has been detected in ALS, suggesting that barrier breakdown is a significant contributor to disease progression [14,15,16,17,18,19,20,21,22,23]. Besides determining systemic biomarkers associated with ALS, it is also important to evaluate detrimental effect(s) of humoral factors on endothelium homeostasis since ECs comprise the first lining of cells separating the blood compartment from CNS tissues [53,54].
Recently, a meta-analysis [55] provided a systematic review of 25 publications regarding blood inflammatory cytokines in ALS patients vs. control subjects. Results showed that the levels of TNF-α, TNF receptor 1, IL-6, IL-1β, IL-8, and vascular endothelial growth factor (VEGF) were significantly higher in ALS patients compared to controls, suggesting that these peripheral inflammatory cytokines might be biomarkers for ALS. Potential diagnostic and/or prognostic biomarkers, particularly in systemic compartment, of disease have been intensively investigated over the last decade. Robelin et al. [56] comprehensively reviewed humoral biomarkers at the molecular and cellular levels relevant to major pathogenic mechanisms contributing to motor neuron degeneration in ALS, such as excitotoxicity, oxidative stress, inflammation, metabolic dysfunction, apoptosis, and axonopathy. However, the authors [56] noted that since no proposed systemic biomarkers have yet translated to a clinical setting, several obstacles must be addressed. The authors proposed that it would be “more appropriate to identify panels of biomarkers, rather than focusing on a single gene, protein, or metabolite”. In agreement with the authors’ remark, we also believe that specification of biomarkers is challenging due to systemic changes during disease progression. Additional factors, such as ALS type (familial or sporadic), anatomical onset of motor neuron impairment (upper, lower, or bulbar), and even age of initial symptoms including gender bias may influence variability of investigated biomarkers. In our opinion, cellular sources of various proteins, which might modify peripheral blood content, should also be taken into account.
Additionally, peripheral immune cells contribute to ALS pathogenesis, potentially reflecting an adaptive immune/inflammatory system response (reviewed in [57,58,59]). In SALS patients, increased CD4+ and decreased CD8+ cell levels as well as significantly reduced CD4+CD25+ regulatory T cells (Treg) and CD14+ monocytes were noted in blood [60]. Decreased numbers of Treg lymphocytes have been shown to correlate with rapid disease progression in patients, potentially indicating immune dysfunction in later ALS stages [61]. In contrast, another study [62] showed significant increases of CD8 cytotoxic T cells and natural killer (NK) cells in blood of ALS patients. Additionally, activated macrophages were observed in blood of SALS patients, which persisted throughout the course of disease [63]. The authors also reported that expression of HLA-DR on CD14+ monocytes was related to the rate of disease progression, suggesting a direct relationship between humoral macrophage activation and ALS disease stage. Also, counts of CD16+ peripheral monocytes [64] and neutrophil/lymphocyte ratio [65] were elevated in ALS patients. Relatively recently, a significant increase of neutrophils and decreased CD4+ T cells and CD16− monocytes were shown in ALS patients’ blood, resulting in an increased ratio of neutrophils to CD16− monocytes (N:M ratio) [66]. The authors suggest that reduction of CD4+ lymphocytes and CD16− monocytes reflects extravasation of these cells from the blood into the tissues and that the N:M ratio might be a helpful marker of disease progression. Interestingly, the study showed changes in T cell and monocyte cell populations including IL-6 production between identical female twins, one of which developed ALS and the other did not [67]. Results demonstrated more abundant serum IL-6 and TNF-α productions by macrophages in addition to the presence of CD8+ effector T cells in the ALS-twin vs. the non-ALS twin, leading to the conclusion that high expression of these toxic cytokines on infiltrating macrophages into ALS tissues might contribute to increased inflammatory response.
Thus, cross-linking between the peripheral immune and inflammatory cell responses in ALS likely indicates the complexity of a dynamic immune/inflammatory system response. The complicated interactions would be not only dependent on the current disease stage but also on the humoral content of specific biomarkers. Since inflammation reflects a cascade of processes underlying particular cellular system responses largely controlled by actions of different mediators released under inflammatory conditions, monitoring the status of inflammatory mediators in the same patient during disease progression might be a more useful strategy.
In partial support of this suggestion, we demonstrated changes in SALS patients’ humoral factors during disease progression [45]. Cytokines and other factors such as nitrite and glutathione (GSH) levels were analyzed in sera from peripheral blood of ALS patients and age-matched control subjects at two visits separated by 6 months. Mainly, significant increases were noted in levels of IL-6 and IL-8 cytokines; IL-1β level was also elevated in sera from SALS patients vs. controls. Also, significantly reduced GSH and elevated nitrite levels were detected in ALS patients in both visits, indicating ongoing oxidative stress likely due to an imbalance caused by excessive generation of pro-oxidants and insufficient anti-oxidant mechanisms [68,69]. However, a significant increase of IL-6 in sera was determined in ALS patients vs. controls at first visit and a drastic reduction of this cytokine to control levels was noted at second visit. Our findings [45], at least, on initial high IL-6 level are supported by a previous report [44] showing increased IL-6 cytokine levels in sera from ALS patients in correlation with disease duration in range of 0.5–3 years. Though, data on IL-6 concentrations was analyzed from a small cohort ALS patients (n = 11), from which undetectable cytokine levels were noted in three patients. Additionally, significantly higher IL-6 levels were found in cerebrospinal fluid from ALS patients than patients with other neurological diseases [70]. However, discrepancies in IL-6 concentrations between two visits of ALS patients in our study [30] may indicate initial humoral inflammatory status and later infiltration of this protein into CNS tissues. Of note, IL-6 can cross the blood-brain barrier [71]. Permeation of IL-6 in addition to extravasation of immune/inflammatory cells (neutrophils, activated monocytes, and T lymphocytes) expressing this cytokine potentially escalate CNS inflammatory response in ALS. However, particular mechanisms of composed IL-6 actions on motor neuron function need to be explored since neurons might also be a source of IL-6 production in the brain [72]. Furthermore, it has been shown that IL-6 increases may be related to the hypoxia experienced by some ALS patients and may not be indicative of inflammatory status [73].
Our study [45] showed not only significant increases of IL-6, but also in IL-8. Although IL-8 levels were elevated in sera of ALS patients at the first visit, a significant decrease of this protein was determined 6 months later. IL-8 is a member of the Cysteine-X-Cysteine (CXC) chemokine family. IL-8 is produced by various cells, including macrophages and endothelial cells [74] and is mainly known as a pro-inflammatory mediator [75]. Increased HLA-DR (a MHC class II cell surface receptor) expression on monocytes and macrophages has been shown in SALS patient blood during disease progression, suggesting a correlation between systemic macrophage activation and ongoing CNS pathogenic processes [63]. Stimulation of human umbilical vein endothelial cells (HUVECs) with IL-8 in vitro increased endothelial cell permeability by downregulation of tight junction proteins [76]. Yet, recombinant human IL-8 enhanced HUVEC survival and proliferation in vitro, inhibited cell apoptosis, induced matrix metalloproteinase (MMP) production, and regulated angiogenesis [77].
Thus, the elevated pro-inflammatory cytokines and other factors found in blood of ALS patients may be imperative mediators inducing inflammatory EC response. Specifically, the IL-6 cytokine is a possible early extrinsic effector leading to EC inflammation and eventual cell degeneration in ALS.
3. IL-6 Cytokine and Its Receptors
The cytokine IL-6 is a multifunctional protein for regulation of metabolic and regenerative cell processes and is secreted by various cells, including ECs. This cytokine is expressed by unstimulated neutrophils and eosinophils in peripheral blood of healthy donors at variable levels [78]. This suggests active contribution of granulocytes to the IL-6 cytokine content in the systemic compartment. It has been shown that elevated expression of both IL-6 mRNA and protein level in unstimulated neutrophils depend on binding affinity of the constant portion of immunoglobulin G (IgG) to cell surface Fc receptors [79], leading to initiation of neutrophil activation. Blood-borne or blood-derived granulocyte IL-6 production can be rapidly up-regulated with granulocyte-macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF), or TNF-α [79,80]. Upon these specific stimuli, initiation of humoral or cellular immune responses may lead to a transition from innate to adaptive immunity. IL-6 is produced by T and B lymphocytes [81] and this cytokine even induced B cell maturation to augment antibody production [82] as well as growth of T cells and differentiation of naïve CD4+ T cells [83,84]. However, production of cytokines by B lymphocytes, in contrast to T cells, depends on B cells activation and differentiation state to plasma cells. Interestingly, it has been shown that IL-6 expressed by B cells upon initiation of immunoglobulin production and TNF-α secretion is associated mainly with cell proliferation [85]. Vazquez et al. [86] comprehensively discussed the role of several cytokines such as interleukins (IL-7, IL-4, IL-6, and IL-10) and interferons (IFN-α, IFN-β, and IFN-γ) on B cell development, survival, differentiation, and proliferation in regulation of antibody-mediating humoral immunity. Based on evidence that cytokine production by B cells is reliant not only on receiving activation stimuli but also on specific immune microenvironment, the authors conclude that B cells are “regulatory cells of the immune system” and “should be considered an integral component of the adaptive immune system” [86].
Peripheral monocytes are also a cellular producer of cytokines, including IL-6 (reviewed in [87]). Upon activation of monocytes and differentiation into macrophages, these cells have protective effect by phagocytizing various foreign substances and pathogens [88,89]. There are three distinct human monocyte subsets based on relative surface expression of co-receptors CD14 and CD16: classical (CD14++/CD16−), non-classical (CD14+/CD16++), and intermediate or transitional (CD14++/CD16+) [90,91,92]. These heterogenic monocyte phenotypes are closely related to their functions [93,94]. Mukherjee et al. [95] reported that “classical” monocytes are primarily phagocytic cells producing IL-10 with no inflammatory actions; “non-classical” monocytes exhibit inflammatory characteristics upon their activation by producing TNF-α and IL-1β and might also act as antigen presenting cells; “intermediate” monocytes, as a minor transitional cell subset, show both phagocytic and inflammatory function. Also, circulating blood monocytes are capable of migrating to an inflamed tissue site, including the CNS, providing defensive effect by phagocytizing host cell debris to lower inflammation and then might exacerbate inflammation by producing excessive amounts of pro-inflammatory cytokines such as TNF-α, IL-1, IL-6, IL-8, and IL-12 [87,96]. Interestingly, tissue-resident macrophages initially promote neutrophil infiltration into inflamed tissue sites followed by extravasation of inflammatory monocytes (reviewed in [96]). During this process, as the authors discussed, neutrophils produce soluble complexes of IL-6 and it receptors, activating endothelial cells to express C-C motif chemokine ligand 2 (CCL2) and vascular cell-adhesion molecule 1 (VCAM1). Extravasation of leukocytes into the CNS tissue is a multistep process: rolling, activation, arrest, crawling, and migration across the endothelial cells via the paracellular or transcellular pathway (reviewed in [97]). Each step of transendothelial cell migration is tightly controlled at the molecular level. For example, adhesion molecules (E-, P-, and L-selectins) mediate leukocyte rolling on activated endothelia in an initial step in the recruitment of leukocytes to an inflammatory site [98]. When human peripheral blood monocytes were cultured in P-selectin-coated plates, the secretion of IL-1β, IL-6, IL-8, IL-12, and macrophage inflammatory protein MIP-1β by monocytes was 10-fold higher compared with unstimulated monocytes after 20 h of culture [99]. Results of this study demonstrated that P-selectin has an important role in monocyte trafficking and cytokine production by these cells.
Additionally, endothelial cells per se have an important role in inflammation by responding to endogenous and exogenous pro-inflammatory stimuli by production of various cytokines, chemokines, and adhesion molecules. In one study [100], HUVEC treated with pro-inflammatory factors such as lipopolysaccharide (LPS), TNF-α, or IL-1β in vitro showed different cell responses to stimuli. High expression of IL-6, IL-8, and E-selectin in cell supernatants was mainly determined by IL-1β induction vs. LPS or TNF-α, leading to the authors’ suggestion that this cytokine has an imperative role in neutrophil recruitment through endothelial cells. However, another early study [101] showed that LPS, TNF, and IL-1α rapidly enhance active IL-6 production by HUVEC in addition to affecting cell proliferation. A different study [102] demonstrated that CD16+, not CD16−, a subset of peripheral blood monocytes producing high levels of IL-6, chemokine CCL2, and MMP-9 upon being cultured with TNF/IFN-γ activated HUVEC expressing CX3CL1 (C-X3-C motif chemokine ligand 1). These results suggest that interaction of CD16+ monocytes with CX3CL1-expressing endothelial cells leads to monocyte extravasation. Also, endothelin, a potent vasoconstrictor hormone produced by endothelial cells, stimulated expression of IL-6 by a rat aortic endothelial cell clone inducing endothelium inflammation [103]. Although results of these studies have important scientific value to determine specific inflammatory inducers in endothelial cell response, combination of various cytokines and other factors should be taken into account to better mimic the microenvironment to which endothelial cells might be exposed. Likewise, using endothelial cells damaged or degenerated due to different diseases might be a useful tool for understanding mechanisms of cell damage.
Additionally, the IL-6 cytokine was found to be produced by muscle contraction and released into the blood, mediating anti-inflammatory effects both systemically and locally in the muscle itself [104]. This muscle-derived IL-6, known as a “myokine” or “exercise factor”, mainly suppressed pro-inflammatory cytokine TNF-α production, induced lipolysis, stimulated cortisol expression, and enhanced muscle glycogen levels [105,106]. Production of the IL-6 myokine during exercise also stimulated anti-inflammatory cytokine IL-1 receptor agonist (IL-1ra) and IL-10 appearance in circulating blood [107,108]. The anti-inflammatory effect of exercise by production of IL-6 myokine has important benefits not only for muscle homeostasis, but also for general health. However, contradictory study results of IL-6 myokine in metabolic regulation or even in muscle function per se, indicating a pleiotropic protein effect, were noted and comprehensively reviewed [109,110]. Also, distinguishing muscle-derived IL-6 from blood-borne IL-6 in the systemic compartment might be difficult.
Together, IL-6 cytokine actions are complex and potentially dependent on the levels of other humoral cytokines, which may regulate peripheral cell-cell interaction and function. Peripheral cell cross-talk might rely not only on production of specific cytokines, but also on cytokine composition and the cytokine network within circulating blood microenvironment under physiological or pathological conditions. For example, anti-inflammatory IL-10 cytokine represses the expression of TNF-α, IL-6, and IL-1 cytokines by activated microphages whereas TGF-β counteracts IL-6′s inflammatory effects (reviewed in [111]). However, the role of IL-6 as a primary or secondary inducer in cell response due to interaction with other cytokines is still unclear.
IL-6 can act as an anti- or pro-inflammatory mediator [49,50,51,52]. Numerous comprehensive reviews [112,113,114,115,116,117] discuss mediation of anti-inflammatory functions of IL-6 by the classic signaling pathway [112,113], whereas pro-inflammatory IL-6 responses are facilitated via the trans-signaling pathway [113,115,117]. In the classic signaling pathway, IL-6 stimulates target cells via binding membrane receptor IL-6R in association with the signaling receptor glycoprotein 130 (gp130) initiating intracellular signaling by activation of JAK and other signal transduction molecules (MAPK, ERK, P13K, STAT) [112,114,115]. However, IL-6R can be released by proteolytic shedding from neutrophils or by secretion from monocytes of an alternatively spliced messenger RNA (mRNA) species as a soluble form (sIL-6R) that can bind IL-6 and form binary IL-6/sIL-6R complex in sera [118,119,120,121]. This complex then binds to gp130 on cell membranes, triggering the intracellular trans-signaling pathway [113,122]. Using this trans-signaling mechanism, IL-6 is able to stimulate cells that lack the endogenous membrane IL-6R. Importantly, sIL-6R, which comprises the extracellular portion of the receptor, binds IL-6 with a similar affinity as the membrane bound IL-6R.
High levels of IL-6 and sIL-6R have been demonstrated in several chronic inflammatory and autoimmune diseases [123,124], suggesting IL-6/sIL-6R complex involvement in the transition from acute to chronic inflammation [125]. It has been shown that IL-6/sIL-6R complex induces a pro-inflammatory response in ECs that express gp130, but not IL-6R [122,126,127]. Also, adding IL-6 to cultured bovine vascular endothelial cells for 21 h substantially increased endothelial permeability by rearranging actin filaments and by changing endothelial cell morphology [128]. Thrombin-activated HUVECs secreted IL-6 in vitro and added exogenous sIL-6R, leading to significantly increased IL-6 and monocyte chemotactic protein-1 (MCP-1) productions [127]. These effects were blocked by anti-IL-6 or anti-sIL-6R monoclonal antibodies, suppressing formation of IL-6/sIL-6R/gp130 complex. Moreover, blockage of IL-6 trans-signaling actions in the brain of bigenic GFAP-IL-6/sgp130 mice significantly reduced vascular changes and BBB leakage in addition to decreasing gliosis and enhancing hippocampal neurogenesis, suggesting that sgp130 blocks trans-signaling thereby lessening detrimental effects of IL-6 in the CNS [129]. In the phase III clinical trial, inhibition of IL-6R with tocilizumab significantly improved disease outcomes in patients with rheumatoid arthritis [130].
However, recent studies demonstrated that IL-6 deficiency does not affect disease outcomes in G93A SOD1 mice modeling ALS [131] and IL-6 blockage with a murine surrogate of tocilizumab revealed deleterious clinical effects in these animals despite a modest anti-inflammatory impact [132]. However, the role of IL-6 pathways upon EC status in ALS is not a focus of these important studies.
4. Effects of ALS Plasma Proteins on Human Bone Marrow Derived Endothelial Cells In Vitro
Plasma samples were obtained from clinically definite sporadic ALS patients and healthy controls during peripheral blood processing for mononuclear cell isolation as described [45,133]. This study was approved by the Institutional Review Board at the University of South Florida (IRB #103861, 31 May 2007). Each participant in the study signed an informed consent form prior to enrollment. Plasma samples from a randomly selected SALS male patient at moderate disease stage (ID #004, Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised (ALSRS-R) 22, 69 years old) and an age-matched male control subject (ID #AB, ALSRS-R 48, 65 years old) were used for our preliminary in vitro studies.
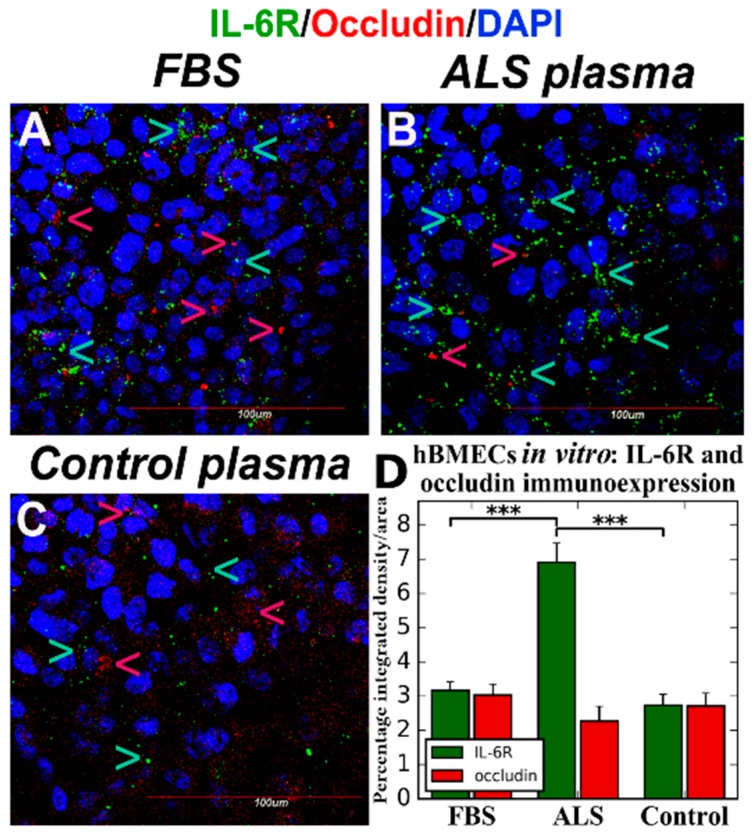
The effect of plasma proteins from the ALS patient and control subject on EC homeostasis and on IL-6R and occludin (tight junction protein) immunoexpressions in ECs was determined using human bone marrow derived endothelial cells (hBMECs, CELPROGEN Inc., Torrance, CA, USA). Initially, hBMECs plated at a density of 5 × 104/mL into 24-well plates in Celprogen Complete Growth Media for 48 h developed a cobblestone morphology and tubular vessel formation. When 10% plasma from the ALS patient was added to culture media, hBMECs demonstrated a disorganized morphology as characterized by swelling, formation of numerous cytoplasmic vesicles, and reduction of cell processes after 48 h. The cells, after adding plasma from the control patient, also displayed cytoplasmic vesicles but morphology such as tubule formation was similar to control cells. These results were supported by our previous report [31] that demonstrated swollen and vacuolated endothelial cells in capillaries of the medulla, cervical, and lumbar spinal cord of post-mortem tissues from SALS.
Follow-up studies were performed using a sensitive BBB in vitro model as described [134]. This BBB model, composed only of hBMECs, allows defining particular molecular mechanism(s) that affect ECs by ALS humoral effectors without influence/interaction from other cellular BBB components. Briefly, a monolayer of hBMECs (105 cells/200 µL) was plated onto a culture insert with semi-permeable membrane (1 µm) in Celprogen Complete Growth Media for 24 h. Then, 10% FBS, plasma from ALS or control patient was added to culture media into an insert compartment. Cells were fixed on a membrane after 5 days in vitro (DIV) and double immunohistochemical staining was performed for IL-6R and occludin using IL-6R mouse monoclonal antibody and rabbit polyclonal anti-occludin antibody, respectively. Then cells were incubated with appropriate secondary antibody conjugated to FITC or rhodamine. Fluorescence immunoexpressions of IL-6R and occludin were analyzed in obtained immunohistochemical images by measuring integrated density of positive cell expression per area using ImageJ software (National Institutes of Health, Bethesda, MD, USA, https://imagej.nih.gov/ij/, version 1.46).
Results showed a significant (p < 0.001) increase of IL-6R immunoexpression in ECs by exposure to ALS plasma (Figure 1B,D) vs. plasma from control patient (Figure 1C,D) or FBS (Figure 1A,D). Also, occludin immunostaining displayed a tendency towards downregulation after adding plasma from an ALS patient (Figure 1D).
Figure 1.
Confocal fluorescent images of hBMECs immunostained for IL-6R and occludin in vitro. Double immunostaining for IL-6R and occludin was performed on fixed hBMECs after 5 DIVexposure to FBS, plasma from ALS or control patient. The cells containing ALS plasma in media demonstrated significantly increased IL-6R (green, arrow) and reduced occludin (red, arrow) immunoexpressions (B,D). There were no differences in IL-6R or occludin immunoexpression between culture cells after exposure to FBS (A) or plasma from control subject (C). DAPI (blue) was used for nuclei staining. Data are presented as means ± S.E.M. Scale bar in (A–C) is 100 µm. *** p < 0.001.
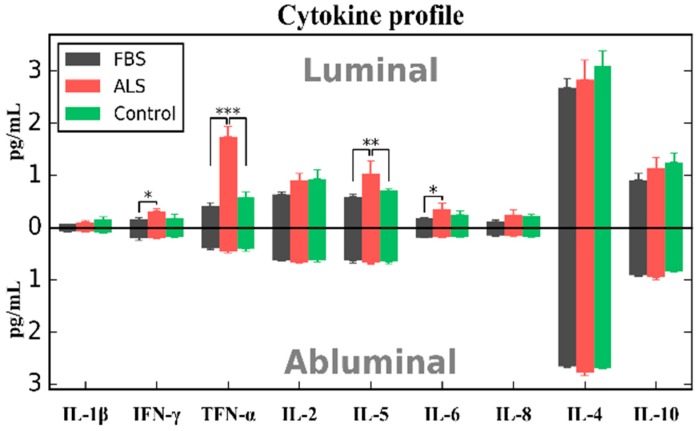
Additionally, cytokine profile (IL-1β, IFN-γ, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, and TNF-α) using multiplex cytokine assay (Thermo Fisher Scientific, Waltham, MA, USA) as described [30] was performed in collected media supernatant from insert (luminal) and 24-well plate (abluminal) compartments prior to hBMEC fixation after 5 DIV of exposure to 10% FBS, plasma from ALS or control patient. Our preliminary data showed significantly elevated concentrations of IFN-γ (p < 0.05), TNF-α (p < 0.001), IL-5 (p < 0.01), and IL-6 (p < 0.05) cytokines at luminal (insert) compartment primarily after cell exposure to ALS plasma (Figure 2). However, significant difference in IFN-γ and IL-6 concentrations was shown only between ALS plasma and FBS exposures. Interestingly, no differences (p > 0.05) in cytokine levels were found between any cell culture conditions at abluminal (24-well plate) side.
Figure 2.
Cytokine profile of media collected from luminal (upper) and abluminal (lower) compartments. Media was collected from luminal (insert, upper) and abluminal (24-well plate, lower) compartments separated by a porous membrane at 5 DIV after exposure to FBS, plasma from ALS or control patient. At luminal side, significant increase of IFN-γ, TNF-α, IL-5, and IL-6 cytokine concentrations were determined at luminal compartment primarily after cell exposure to ALS plasma. There were no differences in cytokine levels between any cell culture conditions at abluminal side of construct. Data are presented as means ± S.E.M. * p < 0.05, ** p < 0.01, *** p < 0.001.
Thus, our preliminary in vitro study results suggest that short term exposure to plasma from ALS patient leads to morphological changes in cultured hBMECs, promotes significant IL-6R immunoexpression, and induces pro-inflammatory cell response. However, since ECs do not express IL-6R [122], detected high expression of these receptors likely reflects cellular IL-6/sIL-6R/gp130 complex formations inducing pro-inflammatory IL-6 trans-signaling, a possibility we are currently investigating. Hypothesizing that humoral IL-6-mediated inflammation triggers EC damage, then restoration of EC integrity with an anti-inflammatory agent(s) preventing IL-6/sIL-6R complex formation or inhibiting membrane trans-signaling sIL-6R/gp130, may afford vascular repair in ALS.
5. Proposed Mechanisms of Humoral IL-6-Mediated Inflammation Triggering Endothelial Cell Damage
We hypothesize that humoral IL-6-mediated inflammation is an early extrinsic effector leading to EC degeneration that principally contributes to B-CNS-B damage in ALS.
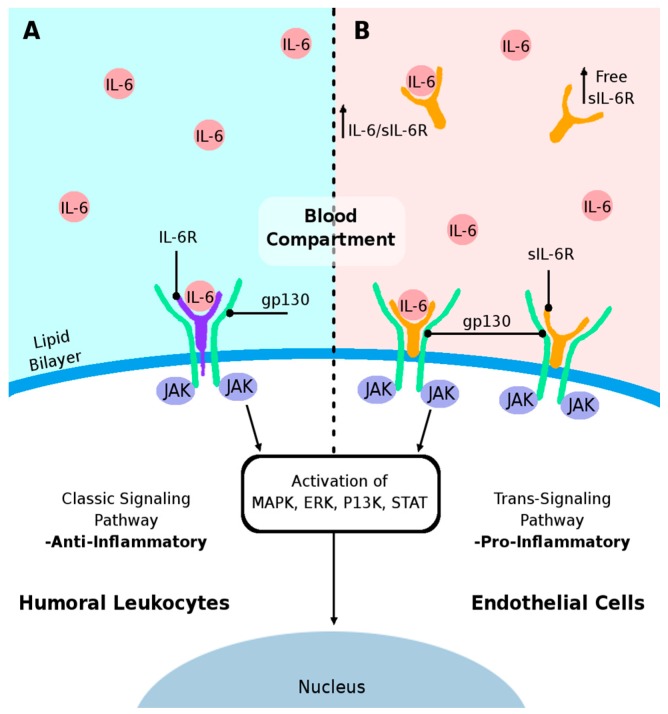
Cytokine IL-6 is a multipotent protein with anti-inflammatory and pro-inflammatory effects. As discussed above, the anti-inflammatory action of IL-6 promotes physiological cell function by binding to the cell membrane receptor IL-6R/gp130 complex via the classic signaling pathway [112,113,114]. In the systemic compartment, leukocyte function might be regulated by various classic intracellular transduction molecules mediating immune/inflammatory cell response in both innate and adaptive immunity (Figure 3A). Elevated IL-6 cytokine levels in blood allow binding of IL-6 to the soluble receptor sIL-6R leading to IL-6/sIL-6R complex formation [118,119,120,121,135]. Upon binding of this complex to gp130 on the cell membrane, a pro-inflammatory cell response is induced by activation of the trans-signaling pathway [113,115] (Figure 3B). Of note, endogenous regulatory mechanisms such as a soluble form of gp130 (sgp130) have been detected in human blood [136,137]. This isoform of sgp130 can bind to the IL-6/sIL-6R complex in the blood circulation and may specifically inhibit IL-6-mediated trans-signaling [113,115].
Figure 3.
Schematic diagram proposed mechanism of humoral IL-6-mediated inflammation in triggering EC damage. (A) The IL-6 cytokine is an important anti-inflammatory protein for regulation of cell survival by binding to the cell membrane receptor IL-6R/gp130 complex leading to activation of JAK, subsequent activation of other signal transduction molecules which influences nuclear gene transcription (down arrow) via the classic signaling pathway; (B) Excessive sIL-6R (up arrow) in the blood, resultant of cleavage from the membrane (shedding) or de novo synthesis, could bind to excess IL-6 and form IL-6/sIL-6R/gp130 complex on ECs. This complex formation on the EC membrane would result in the activation of signal transduction kinases and induce a pro-inflammatory response by activating the trans-signaling pathway.
Accumulated evidence indicates that the humoral immune/inflammatory system response is highly involved in ALS pathogenesis. Potential increase of free sIL-6R is the result of shedding from various humoral cells with primary cellular contributors being neutrophils and activated monocytes. Elevated levels of free sIL-6R promote formation of the IL-6/sIL-6R complex in blood following binding to gp130 on target cell membranes, which activates the trans-signaling pathway and potential induction of pro-inflammatory response in EC. Additionally, activation of this trans-signaling pathway in ECs promotes the de novo synthesis of monocyte-attracting chemokines and vascular cell-adhesion molecules, leading to extravasation of inflammatory or immune cells into the CNS. However, the possibility that the IL-6/sIL-6R-mediated intracellular trans-signaling pathway may induce an EC pro-inflammatory response in ALS warrants investigation.
6. Conclusions and Perspectives
ALS is a complicated incurable disease with multiple etiologies and limited therapies. Numerous factors have been shown to be involved in ALS pathogenesis. One possible effector is blood-CNS barrier impairment, primarily through endothelial cell (EC) degeneration. Although mechanisms of EC damage in ALS remain unidentified, humoral inflammatory factors may initiate disease-related vascular changes. Systemic IL-6-mediated inflammation may be an early extrinsic effector leading to EC death damaging the CNS barrier. Our and other studies showed elevated levels of various inflammatory cytokines, including IL-6, in blood of ALS patients. Excessive humoral IL-6 cytokine levels could induce a pro-inflammatory EC response by activating the trans-signaling pathway, as discussed in this review.
We initiated studies to determine the effect of plasma proteins from ALS patients on EC homeostasis. Our preliminary in vitro studies demonstrated a proof of principle that short term exposure of hBMECs to plasma from ALS patient deteriorates cell morphology and promotes significant IL-6R immunoexpressions, likely reflecting cellular IL-6/sIL-6R/gp130 complex formations. Also, occludin immunostaining displayed a tendency towards downregulation. It is possible that significantly reduced “tightness” between ECs in vitro and increased endothelial permeability could be determined after long term ALS plasma exposure. Additionally, adding ALS plasma at luminal (insert) compartment using BBB in vitro model led to significantly increased IFN-γ, TNF-α, IL-5, and IL-6 cytokine concentrations. These results suggest an induced pro-inflammatory EC response by secretion of specific factors even after brief exposure to humoral proteins from the ALS patient. Yet, cytosolic EC content should confirm inflammatory cell status. Together, our preliminary data showed that EC dysfunction in ALS is potentially initiated by the detrimental humoral inflammatory modulator IL-6 cytokine.
Following our initial study results, our research team will continue to test the hypothesis of humoral IL-6-mediated inflammation triggering EC damage towards the goal of identifying pathogenic mechanism(s) in ALS vascular impairment. For instance, ALS plasma samples obtained at different disease stages and/or increased exposure of ECs in vitro to ALS plasma may be essential to mimic clinical outcomes on EC damage. Since IL-6 induces the trans-signaling inflammatory pathway via soluble receptors in pathophysiological situation, exploring molecular mechanisms of this pathway is important to understand inflammatory EC response in ALS. Primarily, determining free sIL-6R levels and IL-6/sIL-6R complexes in plasma from ALS patients with initial, moderate, and advanced disease stages is imperative to confirm the role of the IL-6 cytokine as a mediator of EC inflammation. Also, membrane trans-signaling sIL-6R/gp130 complex formations on ECs should be resolved after exposure of ALS plasma proteins. Additionally, investigation of the downstream intracellular signaling pathway by involvement of JAK or other signal transduction molecules may be essential to determine EC alterations in ALS.
We are currently performing the above mentioned and other studies, which may form the basis for a therapeutic approach towards vascular repair via an endothelial-IL-6-targeting therapy in ALS. An anti-inflammatory agent for EC restoration could be approached for modulating IL-6 induced inflammation by decreasing sIL-6R levels and IL-6/sIL-6R complex formations or by inhibiting membrane trans-signaling sIL-6R/gp130 in EC membrane. Also, modulation of soluble IL-6R rather than the membrane-bound IL-6R may be a promising approach to regulate and/or control pro-inflammatory effect of IL-6 via suppression of IL-6 trans-signaling activation, leading to EC repair in ALS.
Acknowledgments
This review was supported by the Center of Excellence for Aging and Brain Repair, Department of Neurosurgery, University of South Florida. Svitlana Garbuzova-Davis, Paul R. Sanberg, and Cesario V. Borlongan are funded by NIH 1R01 NS090962-01 grant.
Abbreviations
| ALS | amyotrophic lateral sclerosis |
| ALSRS-R | amyotrophic lateral sclerosis functional rating scale-revised |
| BBB | blood-brain barrier |
| B-CNS-B | blood-CNS barrier |
| BSCB | blood-spinal cord barrier |
| CCL2 | C-C motif chemokine ligand 2 |
| CNS | central nervous system |
| CX3CL1 | C-X3-C motif chemokine ligand 1 |
| CXC | cysteine-X-cysteine |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DIV | days in vitro |
| EC | endothelial cell |
| ERK | extracellular signal-regulated kinase |
| FALS | familial ALS |
| FBS | fetal bovine serum |
| FITC | fluorescein Isothiocyanate |
| G-CSF | granulocyte colony-stimulating factor |
| GM-CSF | granulocyte-macrophage colony-stimulating factor |
| gp130 | glycoprotein 130 |
| GSH | glutathione |
| hBMEC | human bone marrow derived endothelial cell |
| hUVEC | human umbilical vein endothelial cell |
| IFN-β | interferon-beta |
| IFN-γ | interferon-gamma |
| IgG | immunoglobulin G |
| IL | interleukin |
| IL-6R | interleukin-6 receptor |
| JAK | janus kinase |
| LPS | lipopolysaccharide |
| MAPK | mitogen-activated protein kinase |
| MCP-1 | monocyte chemotactic protein-1 |
| MHC | major histocompatibility complex |
| MMP | matrix metalloproteinase |
| mRNA | messenger RNA |
| PI3K | phosphatidyl-inositol-3-kinase |
| SALS | sporadic ALS |
| sgp130 | soluble form of gp130 |
| sIL-6R | soluble form of IL-6R |
| SOD-1 | superoxide dismutase 1 |
| STAT | signal transducer and activator of transcription |
| TGF- β | transforming growth factor-beta |
| TNF-α | tumor necrosis factor-alpha |
Author Contributions
Svitlana Garbuzova-Davis wrote the manuscript. Svitlana Garbuzova-Davis and Cesario V. Borlongan designed the preliminary study. Jared Ehrhart assisted Svitlana Garbuzova-Davis with in vitro studies, cell imaging, and data analysis. Jared Ehrhart drew the schematic diagram of the proposed mechanism for humoral IL-6-mediated EC inflammation. Cesario V. Borlongan and Paul R. Sanberg participated in topic discussions. All authors reviewed the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Kiernan M.C., Vucic S., Cheah B.C., Turner M.R., Eisen A., Hardiman O., Burrell J.R., Zoing M.C. Amyotrophic lateral sclerosis. Lancet. 2011;377:942–955. doi: 10.1016/S0140-6736(10)61156-7. [DOI] [PubMed] [Google Scholar]
- 2.Sorarù G., Ermani M., Logroscino G., Palmieri A., D’Ascenzo C., Orsetti V., Volpe M., Cima V., Zara G., Pegoraro E., et al. Natural history of upper motor neuron-dominant ALS. Amyotroph. Lateral Scler. 2010;11:424–429. doi: 10.3109/17482960903300867. [DOI] [PubMed] [Google Scholar]
- 3.Talbot K. Motor neuron disease: The bare essentials. Pract. Neurol. 2009;9:303–309. doi: 10.1136/jnnp.2009.188151. [DOI] [PubMed] [Google Scholar]
- 4.Logroscino G., Traynor B.J., Hardiman O., Chiò A., Mitchell D., Swingler R.J., Millul A., Benn E., Beghi E. Incidence of amyotrophic lateral sclerosis in Europe. J. Neurol. Neurosurg. Psychiatry. 2010;81:385–390. doi: 10.1136/jnnp.2009.183525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosen D.R., Siddique T., Patterson D., Figlewicz D.A., Sapp P., Hentati A., Donaldson D., Goto J., O’Regan J.P., Deng H.X. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 6.Gurney M.E., Pu H., Chiu A.Y., Dal Canto M.C., Polchow C.Y., Alexander D.D., Caliendo J., Hentati A., Kwon Y.W., Deng H.X. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 7.Pasinelli P., Brown R.H. Molecular biology of amyotrophic lateral sclerosis: Insights from genetics. Nat. Rev. Neurosci. 2006;7:710–723. doi: 10.1038/nrn1971. [DOI] [PubMed] [Google Scholar]
- 8.Taylor J.P., Brown R.H., Cleveland D.W. Decoding ALS: From genes to mechanism. Nature. 2016;539:197–206. doi: 10.1038/nature20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen S., Sayana P., Zhang X., Le W. Genetics of amyotrophic lateral sclerosis: An update. Mol. Neurodegener. 2013;8:28. doi: 10.1186/1750-1326-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeJesus-Hernandez M., Mackenzie I.R., Boeve B.F., Boxer A.L., Baker M., Rutherford N.J., Nicholson A.M., Finch N.A., Flynn H., Adamson J., et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Renton A.E., Majounie E., Waite A., Simón-Sánchez J., Rollinson S., Gibbs J.R., Schymick J.C., Laaksovirta H., van Swieten J.C., Myllykangas L., et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon P.H. Amyotrophic lateral sclerosis: Pathophysiology, diagnosis and management. CNS Drugs. 2011;25:1–15. doi: 10.2165/11586000-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.Gordon P.H. Amyotrophic Lateral Sclerosis: An update for 2013 Clinical Features, Pathophysiology, Management and Therapeutic Trials. Aging Dis. 2013;4:295–310. doi: 10.14336/AD.2013.0400295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rocha J.A., Reis C., Simões F., Fonseca J., Mendes Ribeiro J. Diagnostic investigation and multidisciplinary management in motor neuron disease. J. Neurol. 2005;252:1435–1447. doi: 10.1007/s00415-005-0007-9. [DOI] [PubMed] [Google Scholar]
- 15.Wijesekera L.C., Leigh P.N. Amyotrophic lateral sclerosis. Orphanet J. Rare Dis. 2009;4:3. doi: 10.1186/1750-1172-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goutman S.A. Diagnosis and Clinical Management of Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders. Contin. Lifelong Learn. Neurol. 2017;23:1332–1359. doi: 10.1212/CON.0000000000000535. [DOI] [PubMed] [Google Scholar]
- 17.Calvo A.C., Manzano R., Mendonça D.M.F., Muñoz M.J., Zaragoza P., Osta R. Amyotrophic lateral sclerosis: A focus on disease progression. BioMed Res. Int. 2014;2014:925101. doi: 10.1155/2014/925101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomik J., Tomik B., Gajec S., Ceranowicz P., Pihut M., Olszanecki R., Stręk P., Składzień J. The balloon-based manometry evaluation of swallowing in patients with amyotrophic lateral sclerosis. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18040707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McConnel F.M., Cerenko D., Hersh T., Weil L.J. Evaluation of pharyngeal dysphagia with manofluorography. Dysphagia. 1988;2:187–195. doi: 10.1007/BF02414425. [DOI] [PubMed] [Google Scholar]
- 20.Cleveland D.W., Rothstein J.D. From Charcot to Lou Gehrig: Deciphering selective motor neuron death in ALS. Nat. Rev. Neurosci. 2001;2:806–819. doi: 10.1038/35097565. [DOI] [PubMed] [Google Scholar]
- 21.Strong M.J., Kesavapany S., Pant H.C. The pathobiology of amyotrophic lateral sclerosis: A proteinopathy? J. Neuropathol. Exp. Neurol. 2005;64:649–664. doi: 10.1097/01.jnen.0000173889.71434.ea. [DOI] [PubMed] [Google Scholar]
- 22.Martin L.J., Price A.C., Kaiser A., Shaikh A.Y., Liu Z. Mechanisms for neuronal degeneration in amyotrophic lateral sclerosis and in models of motor neuron death (Review) Int. J. Mol. Med. 2000;5:3–13. doi: 10.3892/ijmm.5.1.3. [DOI] [PubMed] [Google Scholar]
- 23.Pratt A.J., Getzoff E.D., Perry J.J.P. Amyotrophic lateral sclerosis: Update and new developments. Degener. Neurol. Neuromuscul. Dis. 2012;2012:1–14. doi: 10.2147/DNND.S19803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothstein J.D. Current hypotheses for the underlying biology of amyotrophic lateral sclerosis. Ann. Neurol. 2009;65:S3–S9. doi: 10.1002/ana.21543. [DOI] [PubMed] [Google Scholar]
- 25.Hugon J. Riluzole and ALS therapy. Wien. Med. Wochenschr. 1996;146:185–187. [PubMed] [Google Scholar]
- 26.Rothstein J.D. Edaravone: A new drug approved for ALS. Cell. 2017;171:725. doi: 10.1016/j.cell.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 27.Miller R.G., Mitchell J.D., Lyon M., Moore D.H. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND) Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2003;4:191–206. [PubMed] [Google Scholar]
- 28.Garbuzova-Davis S., Saporta S., Sanberg P.R. Implications of blood-brain barrier disruption in ALS. Amyotroph. Lateral Scler. 2008;9:375–376. doi: 10.1080/17482960802160990. [DOI] [PubMed] [Google Scholar]
- 29.Garbuzova-Davis S., Haller E., Saporta S., Kolomey I., Nicosia S.V., Sanberg P.R. Ultrastructure of blood-brain barrier and blood-spinal cord barrier in SOD1 mice modeling ALS. Brain Res. 2007;1157:126–137. doi: 10.1016/j.brainres.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 30.Garbuzova-Davis S., Saporta S., Haller E., Kolomey I., Bennett S.P., Potter H., Sanberg P.R. Evidence of compromised blood-spinal cord barrier in early and late symptomatic SOD1 mice modeling ALS. PLoS ONE. 2007;2:e1205. doi: 10.1371/journal.pone.0001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garbuzova-Davis S., Hernandez-Ontiveros D.G., Rodrigues M.C.O., Haller E., Frisina-Deyo A., Mirtyl S., Sallot S., Saporta S., Borlongan C.V., Sanberg P.R. Impaired blood-brain/spinal cord barrier in ALS patients. Brain Res. 2012;1469:114–128. doi: 10.1016/j.brainres.2012.05.056. [DOI] [PubMed] [Google Scholar]
- 32.Garbuzova-Davis S., Sanberg P.R. Blood-CNS barrier impairment in ALS patients versus an animal model. Front. Cell. Neurosci. 2014;8:21. doi: 10.3389/fncel.2014.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicaise C., Mitrecic D., Demetter P., De Decker R., Authelet M., Boom A., Pochet R. Impaired blood-brain and blood-spinal cord barriers in mutant SOD1-linked ALS rat. Brain Res. 2009;1301:152–162. doi: 10.1016/j.brainres.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 34.Zhong Z., Deane R., Ali Z., Parisi M., Shapovalov Y., O’Banion M.K., Stojanovic K., Sagare A., Boillee S., Cleveland D.W., et al. ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nat. Neurosci. 2008;11:420–422. doi: 10.1038/nn2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyazaki K., Ohta Y., Nagai M., Morimoto N., Kurata T., Takehisa Y., Ikeda Y., Matsuura T., Abe K. Disruption of neurovascular unit prior to motor neuron degeneration in amyotrophic lateral sclerosis. J. Neurosci. Res. 2011;89:718–728. doi: 10.1002/jnr.22594. [DOI] [PubMed] [Google Scholar]
- 36.Henkel J.S., Beers D.R., Wen S., Bowser R., Appel S.H. Decreased mRNA expression of tight junction proteins in lumbar spinal cords of patients with ALS. Neurology. 2009;72:1614–1616. doi: 10.1212/WNL.0b013e3181a41228. [DOI] [PubMed] [Google Scholar]
- 37.Winkler E.A., Sengillo J.D., Sullivan J.S., Henkel J.S., Appel S.H., Zlokovic B.V. Blood-spinal cord barrier breakdown and pericyte reductions in amyotrophic lateral sclerosis. Acta Neuropathol. 2013;125:111–120. doi: 10.1007/s00401-012-1039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sasaki S. Alterations of the blood-spinal cord barrier in sporadic amyotrophic lateral sclerosis. Neuropathology. 2015;35:518–528. doi: 10.1111/neup.12221. [DOI] [PubMed] [Google Scholar]
- 39.Garbuzova-Davis S., Rodrigues M.C.O., Hernandez-Ontiveros D.G., Louis M.K., Willing A.E., Borlongan C.V., Sanberg P.R. Amyotrophic lateral sclerosis: A neurovascular disease. Brain Res. 2011;1398:113–125. doi: 10.1016/j.brainres.2011.04.049. [DOI] [PubMed] [Google Scholar]
- 40.Rodrigues M.C.O., Hernandez-Ontiveros D.G., Louis M.K., Willing A.E., Borlongan C.V., Sanberg P.R., Voltarelli J.C., Garbuzova-Davis S. Neurovascular aspects of amyotrophic lateral sclerosis. Int. Rev. Neurobiol. 2012;102:91–106. doi: 10.1016/B978-0-12-386986-9.00004-1. [DOI] [PubMed] [Google Scholar]
- 41.Fiala M., Chattopadhay M., La Cava A., Tse E., Liu G., Lourenco E., Eskin A., Liu P.T., Magpantay L., Tse S., et al. IL-17A is increased in the serum and in spinal cord CD8 and mast cells of ALS patients. J. Neuroinflamm. 2010;7:76. doi: 10.1186/1742-2094-7-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Italiani P., Carlesi C., Giungato P., Puxeddu I., Borroni B., Bossù P., Migliorini P., Siciliano G., Boraschi D. Evaluating the levels of interleukin-1 family cytokines in sporadic amyotrophic lateral sclerosis. J. Neuroinflamm. 2014;11:94. doi: 10.1186/1742-2094-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitchell R.M., Simmons Z., Beard J.L., Stephens H.E., Connor J.R. Plasma biomarkers associated with ALS and their relationship to iron homeostasis. Muscle Nerve. 2010;42:95–103. doi: 10.1002/mus.21625. [DOI] [PubMed] [Google Scholar]
- 44.Ono S., Hu J., Shimizu N., Imai T., Nakagawa H. Increased interleukin-6 of skin and serum in amyotrophic lateral sclerosis. J. Neurol. Sci. 2001;187:27–34. doi: 10.1016/S0022-510X(01)00514-7. [DOI] [PubMed] [Google Scholar]
- 45.Ehrhart J., Smith A.J., Kuzmin-Nichols N., Zesiewicz T.A., Jahan I., Shytle R.D., Kim S.-H., Sanberg C.D., Vu T.H., Gooch C.L., et al. Humoral factors in ALS patients during disease progression. J. Neuroinflamm. 2015;12:127. doi: 10.1186/s12974-015-0350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Evans M.C., Couch Y., Sibson N., Turner M.R. Inflammation and neurovascular changes in amyotrophic lateral sclerosis. Mol. Cell. Neurosci. 2013;53:34–41. doi: 10.1016/j.mcn.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 47.Su X.W., Simmons Z., Mitchell R.M., Kong L., Stephens H.E., Connor J.R. Biomarker-based predictive models for prognosis in amyotrophic lateral sclerosis. JAMA Neurol. 2013;70:1505–1511. doi: 10.1001/jamaneurol.2013.4646. [DOI] [PubMed] [Google Scholar]
- 48.Brat D.J., Bellail A.C., Van Meir E.G. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro-Oncology. 2005;7:122–133. doi: 10.1215/S1152851704001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fattori E., Cappelletti M., Costa P., Sellitto C., Cantoni L., Carelli M., Faggioni R., Fantuzzi G., Ghezzi P., Poli V. Defective inflammatory response in interleukin 6-deficient mice. J. Exp. Med. 1994;180:1243–1250. doi: 10.1084/jem.180.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tilg H., Dinarello C.A., Mier J.W. IL-6 and APPs: Anti-inflammatory and immunosuppressive mediators. Immunol. Today. 1997;18:428–432. doi: 10.1016/S0167-5699(97)01103-1. [DOI] [PubMed] [Google Scholar]
- 51.Xing Z., Gauldie J., Cox G., Baumann H., Jordana M., Lei X.F., Achong M.K. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J. Clin. Investig. 1998;101:311–320. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dann S.M., Spehlmann M.E., Hammond D.C., Iimura M., Hase K., Choi L.J., Hanson E., Eckmann L. IL-6-dependent mucosal protection prevents establishment of a microbial niche for attaching/effacing lesion-forming enteric bacterial pathogens. J. Immunol. 2008;180:6816–6826. doi: 10.4049/jimmunol.180.10.6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abbott N.J., Patabendige A.A.K., Dolman D.E.M., Yusof S.R., Begley D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 54.Pardridge W.M. Blood-brain barrier biology and methodology. J. Neurovirol. 1999;5:556–569. doi: 10.3109/13550289909021285. [DOI] [PubMed] [Google Scholar]
- 55.Hu Y., Cao C., Qin X.-Y., Yu Y., Yuan J., Zhao Y., Cheng Y. Increased peripheral blood inflammatory cytokine levels in amyotrophic lateral sclerosis: A meta-analysis study. Sci. Rep. 2017;7:9094. doi: 10.1038/s41598-017-09097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robelin L., Gonzalez De Aguilar J.L. Blood biomarkers for amyotrophic lateral sclerosis: Myth or reality? BioMed Res. Int. 2014;2014:525097. doi: 10.1155/2014/525097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodrigues M.C.O., Voltarelli J.C., Sanberg P.R., Borlongan C.V., Garbuzova-Davis S. Immunological aspects in amyotrophic lateral sclerosis. Transl. Stroke Res. 2012;3:331–340. doi: 10.1007/s12975-012-0177-6. [DOI] [PubMed] [Google Scholar]
- 58.Rodrigues M.C.O., Sanberg P.R., Cruz L.E., Garbuzova-Davis S. The innate and adaptive immunological aspects in neurodegenerative diseases. J. Neuroimmunol. 2014;269:1–8. doi: 10.1016/j.jneuroim.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 59.Zhao W., Beers D.R., Appel S.H. Immune-mediated mechanisms in the pathoprogression of amyotrophic lateral sclerosis. J. Neuroimmune Pharmacol. 2013;8:888–899. doi: 10.1007/s11481-013-9489-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mantovani S., Garbelli S., Pasini A., Alimonti D., Perotti C., Melazzini M., Bendotti C., Mora G. Immune system alterations in sporadic amyotrophic lateral sclerosis patients suggest an ongoing neuroinflammatory process. J. Neuroimmunol. 2009;210:73–79. doi: 10.1016/j.jneuroim.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 61.Beers D.R., Henkel J.S., Zhao W., Wang J., Huang A., Wen S., Liao B., Appel S.H. Endogenous regulatory T lymphocytes ameliorate amyotrophic lateral sclerosis in mice and correlate with disease progression in patients with amyotrophic lateral sclerosis. Brain J. Neurol. 2011;134:1293–1314. doi: 10.1093/brain/awr074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rentzos M., Evangelopoulos E., Sereti E., Zouvelou V., Marmara S., Alexakis T., Evdokimidis I. Alterations of T cell subsets in ALS: A systemic immune activation? Acta Neurol. Scand. 2012;125:260–264. doi: 10.1111/j.1600-0404.2011.01528.x. [DOI] [PubMed] [Google Scholar]
- 63.Zhang R., Gascon R., Miller R.G., Gelinas D.F., Mass J., Hadlock K., Jin X., Reis J., Narvaez A., McGrath M.S. Evidence for systemic immune system alterations in sporadic amyotrophic lateral sclerosis (sALS) J. Neuroimmunol. 2005;159:215–224. doi: 10.1016/j.jneuroim.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 64.Schubert W., Schwan H. Detection by 4-parameter microscopic imaging and increase of rare mononuclear blood leukocyte types expressing the Fc gamma RIII receptor (CD16) for immunoglobulin G in human sporadic amyotrophic lateral sclerosis (ALS) Neurosci. Lett. 1995;198:29–32. doi: 10.1016/0304-3940(95)11956-W. [DOI] [PubMed] [Google Scholar]
- 65.Keizman D., Rogowski O., Berliner S., Ish-Shalom M., Maimon N., Nefussy B., Artamonov I., Drory V.E. Low-grade systemic inflammation in patients with amyotrophic lateral sclerosis. Acta Neurol. Scand. 2009;119:383–389. doi: 10.1111/j.1600-0404.2008.01112.x. [DOI] [PubMed] [Google Scholar]
- 66.Murdock B.J., Bender D.E., Kashlan S.R., Figueroa-Romero C., Backus C., Callaghan B.C., Goutman S.A., Feldman E.L. Increased ratio of circulating neutrophils to monocytes in amyotrophic lateral sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2016;3:e242. doi: 10.1212/NXI.0000000000000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lam L., Chin L., Halder R.C., Sagong B., Famenini S., Sayre J., Montoya D., Rubbi L., Pellegrini M., Fiala M. Epigenetic changes in T-cell and monocyte signatures and production of neurotoxic cytokines in ALS patients. FASEB J. 2016;30:3461–3473. doi: 10.1096/fj.201600259RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sies H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997;82:291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 69.D’Amico E., Factor-Litvak P., Santella R.M., Mitsumoto H. Clinical perspective on oxidative stress in sporadic amyotrophic lateral sclerosis. Free Radic. Biol. Med. 2013;65:509–527. doi: 10.1016/j.freeradbiomed.2013.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sekizawa T., Openshaw H., Ohbo K., Sugamura K., Itoyama Y., Niland J.C. Cerebrospinal fluid interleukin 6 in amyotrophic lateral sclerosis: Immunological parameter and comparison with inflammatory and non-inflammatory central nervous system diseases. J. Neurol. Sci. 1998;154:194–199. doi: 10.1016/S0022-510X(97)00228-1. [DOI] [PubMed] [Google Scholar]
- 71.Banks W.A., Kastin A.J., Gutierrez E.G. Penetration of interleukin-6 across the murine blood-brain barrier. Neurosci. Lett. 1994;179:53–56. doi: 10.1016/0304-3940(94)90933-4. [DOI] [PubMed] [Google Scholar]
- 72.Ringheim G.E., Burgher K.L., Heroux J.A. Interleukin-6 mRNA expression by cortical neurons in culture: Evidence for neuronal sources of interleukin-6 production in the brain. J. Neuroimmunol. 1995;63:113–123. doi: 10.1016/0165-5728(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 73.Moreau C., Devos D., Brunaud-Danel V., Defebvre L., Perez T., Destée A., Tonnel A.B., Lassalle P., Just N. Elevated IL-6 and TNF-α levels in patients with ALS: Inflammation or hypoxia? Neurology. 2005;65:1958–1960. doi: 10.1212/01.wnl.0000188907.97339.76. [DOI] [PubMed] [Google Scholar]
- 74.Utgaard J.O., Jahnsen F.L., Bakka A., Brandtzaeg P., Haraldsen G. Rapid secretion of prestored interleukin 8 from Weibel-Palade bodies of microvascular endothelial cells. J. Exp. Med. 1998;188:1751–1756. doi: 10.1084/jem.188.9.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baggiolini M., Clark-Lewis I. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett. 1992;307:97–101. doi: 10.1016/0014-5793(92)80909-Z. [DOI] [PubMed] [Google Scholar]
- 76.Yu H., Huang X., Ma Y., Gao M., Wang O., Gao T., Shen Y., Liu X. Interleukin-8 regulates endothelial permeability by down-regulation of tight junction but not dependent on integrins induced focal adhesions. Int. J. Biol. Sci. 2013;9:966–979. doi: 10.7150/ijbs.6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li A., Dubey S., Varney M.L., Dave B.J., Singh R.K. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J. Immunol. 2003;170:3369–3376. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- 78.Melani C., Mattia G.F., Silvani A., Carè A., Rivoltini L., Parmiani G., Colombo M.P. Interleukin-6 expression in human neutrophil and eosinophil peripheral blood granulocytes. Blood. 1993;81:2744–2749. [PubMed] [Google Scholar]
- 79.Ericson S.G., Zhao Y., Gao H., Miller K.L., Gibson L.F., Lynch J.P., Landreth K.S. Interleukin-6 production by human neutrophils after Fc-receptor cross-linking or exposure to granulocyte colony-stimulating factor. Blood. 1998;91:2099–2107. [PubMed] [Google Scholar]
- 80.Cicco N.A., Lindemann A., Content J., Vandenbussche P., Lübbert M., Gauss J., Mertelsmann R., Herrmann F. Inducible production of interleukin-6 by human polymorphonuclear neutrophils: Role of granulocyte-macrophage colony-stimulating factor and tumor necrosis factor-α. Blood. 1990;75:2049–2052. [PubMed] [Google Scholar]
- 81.Horii Y., Muraguchi A., Suematsu S., Matsuda T., Yoshizaki K., Hirano T., Kishimoto T. Regulation of BSF-2/IL-6 production by human mononuclear cells. Macrophage-dependent synthesis of BSF-2/IL-6 by T cells. J. Immunol. 1988;141:1529–1535. [PubMed] [Google Scholar]
- 82.Hirano T., Yasukawa K., Harada H., Taga T., Watanabe Y., Matsuda T., Kashiwamura S., Nakajima K., Koyama K., Iwamatsu A. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986;324:73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- 83.Lotz M., Jirik F., Kabouridis P., Tsoukas C., Hirano T., Kishimoto T., Carson D.A. B cell stimulating factor 2/interleukin 6 is a costimulant for human thymocytes and T lymphocytes. J. Exp. Med. 1988;167:1253–1258. doi: 10.1084/jem.167.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tanaka T., Narazaki M., Kishimoto T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014;6 doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rieckmann P., Tuscano J.M., Kehrl J.H. Tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) in B-lymphocyte function. Methods. 1997;11:128–132. doi: 10.1006/meth.1996.0396. [DOI] [PubMed] [Google Scholar]
- 86.Vazquez M.I., Catalan-Dibene J., Zlotnik A. B cells responses and cytokine production are regulated by their immune microenvironment. Cytokine. 2015;74:318–326. doi: 10.1016/j.cyto.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arango Duque G., Descoteaux A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014;5:491. doi: 10.3389/fimmu.2014.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chow A., Brown B.D., Merad M. Studying the mononuclear phagocyte system in the molecular age. Nat. Rev. Immunol. 2011;11:788–798. doi: 10.1038/nri3087. [DOI] [PubMed] [Google Scholar]
- 89.Hume D.A. The mononuclear phagocyte system. Curr. Opin. Immunol. 2006;18:49–53. doi: 10.1016/j.coi.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 90.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: Their role in infection and inflammation. J. Leukoc. Biol. 2007;81:584–592. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 91.Ziegler-Heitbrock L., Ancuta P., Crowe S., Dalod M., Grau V., Hart D.N., Leenen P.J.M., Liu Y.-J., MacPherson G., Randolph G.J., et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–e80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 92.Clanchy F.I.L., Holloway A.C., Lari R., Cameron P.U., Hamilton J.A. Detection and properties of the human proliferative monocyte subpopulation. J. Leukoc. Biol. 2006;79:757–766. doi: 10.1189/jlb.0905522. [DOI] [PubMed] [Google Scholar]
- 93.Ziegler-Heitbrock L. Monocyte subsets in man and other species. Cell. Immunol. 2014;289:135–139. doi: 10.1016/j.cellimm.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 94.Grage-Griebenow E., Flad H.D., Ernst M. Heterogeneity of human peripheral blood monocyte subsets. J. Leukoc. Biol. 2001;69:11–20. [PubMed] [Google Scholar]
- 95.Mukherjee R., Kanti Barman P., Kumar Thatoi P., Tripathy R., Kumar Das B., Ravindran B. Non-Classical monocytes display inflammatory features: Validation in sepsis and systemic lupus erythematous. Sci. Rep. 2015;5:13886. doi: 10.1038/srep13886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Soehnlein O., Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat. Rev. Immunol. 2010;10:427–439. doi: 10.1038/nri2779. [DOI] [PubMed] [Google Scholar]
- 97.Takeshita Y., Ransohoff R.M. Inflammatory cell trafficking across the blood-brain barrier: Chemokine regulation and in vitro models. Immunol. Rev. 2012;248:228–239. doi: 10.1111/j.1600-065X.2012.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tedder T.F., Steeber D.A., Chen A., Engel P. The selectins: Vascular adhesion molecules. FASEB J. 1995;9:866–873. doi: 10.1096/fasebj.9.10.7542213. [DOI] [PubMed] [Google Scholar]
- 99.Suzuki J., Hamada E., Shodai T., Kamoshida G., Kudo S., Itoh S., Koike J., Nagata K., Irimura T., Tsuji T. Cytokine secretion from human monocytes potentiated by P-selectin-mediated cell adhesion. Int. Arch. Allergy Immunol. 2013;160:152–160. doi: 10.1159/000339857. [DOI] [PubMed] [Google Scholar]
- 100.Makó V., Czúcz J., Weiszhár Z., Herczenik E., Matkó J., Prohászka Z., Cervenak L. Proinflammatory activation pattern of human umbilical vein endothelial cells induced by IL-1β, TNF-α, and LPS. Cytom. Part A. 2010;77:962–970. doi: 10.1002/cyto.a.20952. [DOI] [PubMed] [Google Scholar]
- 101.May L.T., Torcia G., Cozzolino F., Ray A., Tatter S.B., Santhanam U., Sehgal P.B., Stern D. Interleukin-6 gene expression in human endothelial cells: RNA start sites, multiple IL-6 proteins and inhibition of proliferation. Biochem. Biophys. Res. Commun. 1989;159:991–998. doi: 10.1016/0006-291X(89)92206-7. [DOI] [PubMed] [Google Scholar]
- 102.Ancuta P., Wang J., Gabuzda D. CD16+ monocytes produce IL-6, CCL2, and matrix metalloproteinase-9 upon interaction with CX3CL1-expressing endothelial cells. J. Leukoc. Biol. 2006;80:1156–1164. doi: 10.1189/jlb.0206125. [DOI] [PubMed] [Google Scholar]
- 103.Xin X., Cai Y., Matsumoto K., Agui T. Endothelin-induced interleukin-6 production by rat aortic endothelial cells. Endocrinology. 1995;136:132–137. doi: 10.1210/endo.136.1.7828523. [DOI] [PubMed] [Google Scholar]
- 104.Brandt C., Pedersen B.K. The role of exercise-induced myokines in muscle homeostasis and the defense against chronic diseases. J. Biomed. Biotechnol. 2010;2010:520258. doi: 10.1155/2010/520258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pedersen B.K., Steensberg A., Fischer C., Keller C., Keller P., Plomgaard P., Wolsk-Petersen E., Febbraio M. The metabolic role of IL-6 produced during exercise: Is IL-6 an exercise factor? Proc. Nutr. Soc. 2004;63:263–267. doi: 10.1079/PNS2004338. [DOI] [PubMed] [Google Scholar]
- 106.Pedersen B.K., Fischer C.P. Beneficial health effects of exercise—The role of IL-6 as a myokine. Trends Pharmacol. Sci. 2007;28:152–156. doi: 10.1016/j.tips.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 107.Petersen A.M.W., Pedersen B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2005;98:1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 108.Steensberg A., Fischer C.P., Keller C., Møller K., Pedersen B.K. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am. J. Physiol. Endocrinol. Metab. 2003;285:E433–E437. doi: 10.1152/ajpendo.00074.2003. [DOI] [PubMed] [Google Scholar]
- 109.Pal M., Febbraio M.A., Whitham M. From cytokine to myokine: The emerging role of interleukin-6 in metabolic regulation. Immunol. Cell Biol. 2014;92:331–339. doi: 10.1038/icb.2014.16. [DOI] [PubMed] [Google Scholar]
- 110.Muñoz-Cánoves P., Scheele C., Pedersen B.K., Serrano A.L. Interleukin-6 myokine signaling in skeletal muscle: A double-edged sword? FEBS J. 2013;280:4131–4148. doi: 10.1111/febs.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang J.-M., An J. Cytokines, inflammation and pain. Int. Anesthesiol. Clin. 2007;45:27–37. doi: 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Scheller J., Rose-John S. Interleukin-6 and its receptor: From bench to bedside. Med. Microbiol. Immunol. 2006;195:173–183. doi: 10.1007/s00430-006-0019-9. [DOI] [PubMed] [Google Scholar]
- 113.Scheller J., Chalaris A., Schmidt-Arras D., Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 114.Wolf J., Rose-John S., Garbers C. Interleukin-6 and its receptors: A highly regulated and dynamic system. Cytokine. 2014;70:11–20. doi: 10.1016/j.cyto.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 115.Rose-John S. IL-6 trans-signaling via the soluble IL-6 receptor: Importance for the pro-inflammatory activities of IL-6. Int. J. Biol. Sci. 2012;8:1237–1247. doi: 10.7150/ijbs.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Van Snick J. Interleukin-6: An overview. Annu. Rev. Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- 117.Barnes T.C., Anderson M.E., Moots R.J. The many faces of interleukin-6: The role of IL-6 in inflammation, vasculopathy, and fibrosis in systemic sclerosis. Int. J. Rheumatol. 2011;2011:721608. doi: 10.1155/2011/721608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Müllberg J., Schooltink H., Stoyan T., Günther M., Graeve L., Buse G., Mackiewicz A., Heinrich P.C., Rose-John S. The soluble interleukin-6 receptor is generated by shedding. Eur. J. Immunol. 1993;23:473–480. doi: 10.1002/eji.1830230226. [DOI] [PubMed] [Google Scholar]
- 119.Horiuchi S., Koyanagi Y., Zhou Y., Miyamoto H., Tanaka Y., Waki M., Matsumoto A., Yamamoto M., Yamamoto N. Soluble interleukin-6 receptors released from T cell or granulocyte/macrophage cell lines and human peripheral blood mononuclear cells are generated through an alternative splicing mechanism. Eur. J. Immunol. 1994;24:1945–1948. doi: 10.1002/eji.1830240837. [DOI] [PubMed] [Google Scholar]
- 120.Peters M., Jacobs S., Ehlers M., Vollmer P., Müllberg J., Wolf E., Brem G., Meyer zum Büschenfelde K.H., Rose-John S. The function of the soluble interleukin 6 (IL-6) receptor in vivo: Sensitization of human soluble IL-6 receptor transgenic mice towards IL-6 and prolongation of the plasma half-life of IL-6. J. Exp. Med. 1996;183:1399–1406. doi: 10.1084/jem.183.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Leggate M., Nowell M.A., Jones S.A., Nimmo M.A. The response of interleukin-6 and soluble interleukin-6 receptor isoforms following intermittent high intensity and continuous moderate intensity cycling. Cell Stress Chaperones. 2010;15:827–833. doi: 10.1007/s12192-010-0192-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Romano M., Sironi M., Toniatti C., Polentarutti N., Fruscella P., Ghezzi P., Faggioni R., Luini W., van Hinsbergh V., Sozzani S., et al. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity. 1997;6:315–325. doi: 10.1016/S1074-7613(00)80334-9. [DOI] [PubMed] [Google Scholar]
- 123.Jones S.A., Richards P.J., Scheller J., Rose-John S. IL-6 transsignaling: The in vivo consequences. J. Interferon Cytokine Res. 2005;25:241–253. doi: 10.1089/jir.2005.25.241. [DOI] [PubMed] [Google Scholar]
- 124.Rose-John S., Waetzig G.H., Scheller J., Grötzinger J., Seegert D. The IL-6/sIL-6R complex as a novel target for therapeutic approaches. Expert Opin. Ther. Targets. 2007;11:613–624. doi: 10.1517/14728222.11.5.613. [DOI] [PubMed] [Google Scholar]
- 125.Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res. Ther. 2006;8(Suppl. 2):S3. doi: 10.1186/ar1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Modur V., Li Y., Zimmerman G.A., Prescott S.M., McIntyre T.M. Retrograde inflammatory signaling from neutrophils to endothelial cells by soluble interleukin-6 receptor α. J. Clin. Investig. 1997;100:2752–2756. doi: 10.1172/JCI119821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Marin V., Montero-Julian F.A., Grès S., Boulay V., Bongrand P., Farnarier C., Kaplanski G. The IL-6-soluble IL-6Rα autocrine loop of endothelial activation as an intermediate between acute and chronic inflammation: An experimental model involving thrombin. J. Immunol. 2001;167:3435–3442. doi: 10.4049/jimmunol.167.6.3435. [DOI] [PubMed] [Google Scholar]
- 128.Maruo N., Morita I., Shirao M., Murota S. IL-6 increases endothelial permeability in vitro. Endocrinology. 1992;131:710–714. doi: 10.1210/endo.131.2.1639018. [DOI] [PubMed] [Google Scholar]
- 129.Campbell I.L., Erta M., Lim S.L., Frausto R., May U., Rose-John S., Scheller J., Hidalgo J. Trans-signaling is a dominant mechanism for the pathogenic actions of interleukin-6 in the brain. J. Neurosci. 2014;34:2503–2513. doi: 10.1523/JNEUROSCI.2830-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Emery P., Keystone E., Tony H.P., Cantagrel A., van Vollenhoven R., Sanchez A., Alecock E., Lee J., Kremer J. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: Results from a 24-week multicentre randomised placebo-controlled trial. Ann. Rheum. Dis. 2008;67:1516–1523. doi: 10.1136/ard.2008.092932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Han Y., Ripley B., Serada S., Naka T., Fujimoto M. Interleukin-6 deficiency does not affect motor neuron disease caused by superoxide dismutase 1 mutation. PLoS ONE. 2016;11:e0153399. doi: 10.1371/journal.pone.0153399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Patin F., Baranek T., Vourc’h P., Nadal-Desbarats L., Goossens J.-F., Marouillat S., Dessein A.-F., Descat A., Hounoum B.M., Bruno C., et al. Combined metabolomics and transcriptomics approaches to assess the IL-6 blockade as a therapeutic of ALS: Deleterious alteration of lipid metabolism. Neurother. J. Am. Soc. Exp. Neurother. 2016;13:905–917. doi: 10.1007/s13311-016-0461-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Saleh I.A., Zesiewicz T., Xie Y., Sullivan K.L., Miller A.M., Kuzmin-Nichols N., Sanberg P.R., Garbuzova-Davis S. Evaluation of humoral immune response in adaptive immunity in ALS patients during disease progression. J. Neuroimmunol. 2009;215:96–101. doi: 10.1016/j.jneuroim.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 134.Paradis A., Leblanc D., Dumais N. Optimization of an in vitro human blood-brain barrier model: Application to blood monocyte transmigration assays. MethodsX. 2016;3:25–34. doi: 10.1016/j.mex.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Müllberg J., Dittrich E., Graeve L., Gerhartz C., Yasukawa K., Taga T., Kishimoto T., Heinrich P.C., Rose-John S. Differential shedding of the two subunits of the interleukin-6 receptor. FEBS Lett. 1993;332:174–178. doi: 10.1016/0014-5793(93)80507-Q. [DOI] [PubMed] [Google Scholar]
- 136.Narazaki M., Yasukawa K., Saito T., Ohsugi Y., Fukui H., Koishihara Y., Yancopoulos G.D., Taga T., Kishimoto T. Soluble forms of the interleukin-6 signal-transducing receptor component gp130 in human serum possessing a potential to inhibit signals through membrane-anchored gp130. Blood. 1993;82:1120–1126. [PubMed] [Google Scholar]
- 137.Zhang J.G., Zhang Y., Owczarek C.M., Ward L.D., Moritz R.L., Simpson R.J., Yasukawa K., Nicola N.A. Identification and characterization of two distinct truncated forms of gp130 and a soluble form of leukemia inhibitory factor receptor α-chain in normal human urine and plasma. J. Biol. Chem. 1998;273:10798–10805. doi: 10.1074/jbc.273.17.10798. [DOI] [PubMed] [Google Scholar]