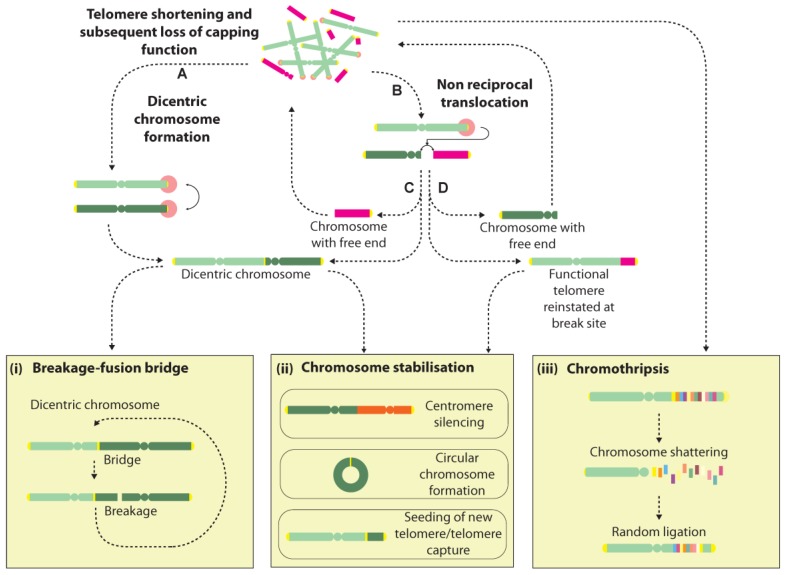
Figure 2.
Cascades of genome rearrangement induced by free chromosome ends. Telomeres that lose their capping function can trigger DNA damage signalling and subsequent processing by DSB repair pathways, instigating genome instability via several mechanisms. Telomere-telomere (A) fusions create dicentric chromosomes that may undergo rupture during cell division, giving rise to a succession of events in a BFB cycle (i). Alternatively, dicentric chromosomes may be stabilised by centromere silencing (ii). Dysfunctional telomeres may also undergo joining with internal genomic loci giving rise to non-reciprocal translocations (B). Depending on the nature of the join, several outcomes are possible with examples of events given in C&D. One outcome is the formation of a dicentric chromosome plus an acentric chromosome (C), which may elicit further BFB events or instability by reintegration of the acentric chromosome at another genomic site. Alternatively, a functional telomere may be transferred to the initiating chromosome, at the cost of creating another free end at the participating chromosome (D). Chromosomes with free ends may be stabilised by circularisation, or through the seeding of a new telomere (ii). Recent reports suggest that telomere dysfunction may also trigger “all-at-once” events such as chromothripsis, a process thought to involve the shattering and random re-ligation of chromosomes that may either give rise to a stable genomic configuration, or may promote further instability due to the presence of a free chromosome end (iii).