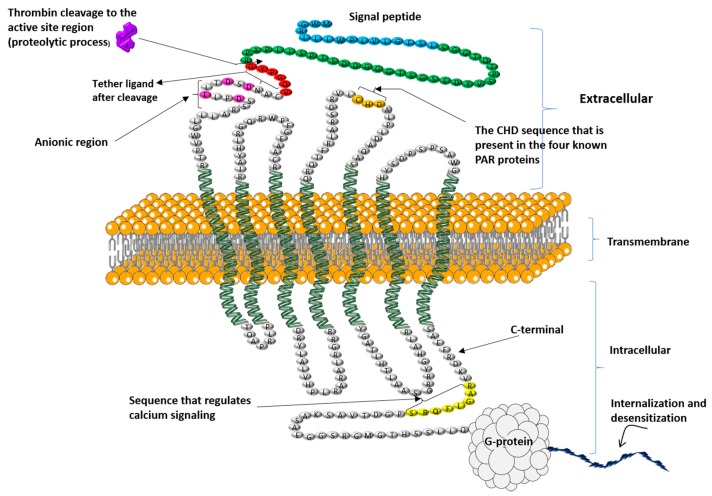
Figure 1.
The proposed structure of human PAR4 with its different parts and their functions. Thrombin cleavage site that reveals a neo-amino-terminus (showed by arrow), the tethered ligand sequence that binds to the second intracellular loop after thrombin cleavage (sequence with the red color), and the Cysteine, Histidine, Aspartic Acid (CHD) amino acids sequence that is present in the four known PAR proteins (gold color), the RAGLFQRS sequence (showed by yellow color) discovered by Ramachandran and colleagues [17] for its role in regulating calcium signaling and interactions of the receptor with β-arrestins (1 and 2), and the anionic region that play a role of interacting with thrombin exosite-I (showed by rose color), and the signal peptide (showed by blue color), the amino-terminal peptide cleaved by thrombin (showed by green color), then the remaining extracellular and intracellular regions are shown in gray.