Table 2. Scope of electrophilic carbonyl coupling partners a , b .
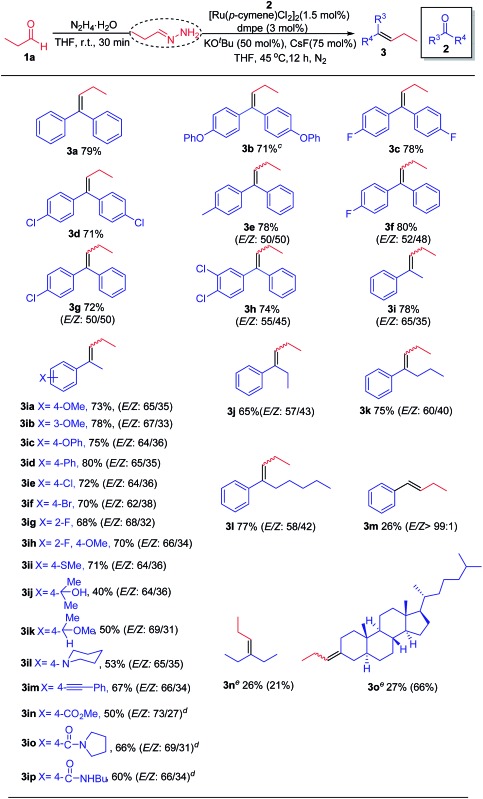
|
a 1a (0.28 mmol, 1.4 equiv.), N2H4·H2O (0.3 mmol, 1.5 equiv.), THF (0.14 mL), rt, 30 min; 2 (0.20 mmol, 1.0 equiv.), [Ru(p-cymene)Cl2]2 (0.003 mmol, 1.5 mol%), dmpe (0.006 mmol, 3.0 mol%), KOtBu (0.1 mmol, 50 mol%), CsF (0.15 mmol, 75 mol%), 45 °C, 12 h, under N2.
bIsolated yields and the ratio of E/Z isomers were determined by crude 1H NMR analysis.
c80 °C.
dK3PO4 (0.1 mmol, 50 mol%), 12 h.
eYields were determined by crude 1H NMR using mesitylene as an internal standard. Yields of asymmetric azines were given in the parentheses.
