Fig. 1.
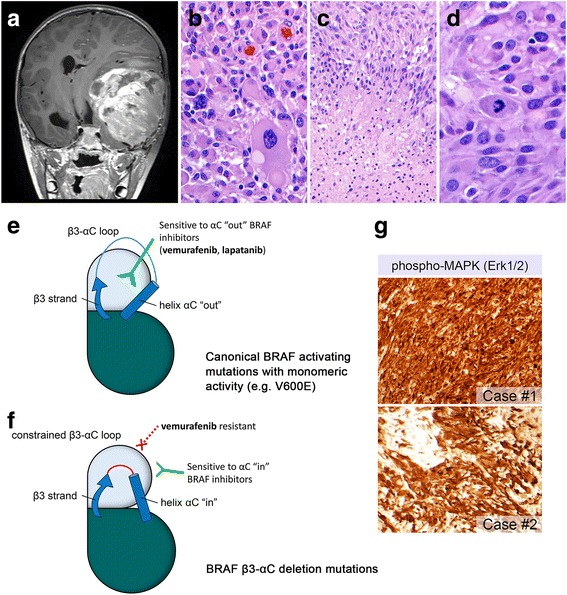
A-PXA with non-V600E activating mutations affecting the β3-αC loop in BRAF. Post-contrast T1-weighted coronal MR sequence showing a large space-occupying lesion with significant midline shift (a). Histologic sections from case #1 demonstrated pleomorphic giant cells (b), as well as pseudopalisading necrosis (c) and increased mitotic activity (d). Illustration of conformational changes of the β3 strand and αC helix in the kinase domain. The canonical BRAF V600E mutation results in monomeric activity and can accommodate the oncogenic BRAF inhibitor vemurafenib, which only binds when helix αC is “out” (e). In β3-αC deletion mutations, the β3-αC loop is shortened, effectively locking helix αC in the “in” position and conferring resistance to vemurafenib (f) (modified with permission from Trends in Cancer, 2 (12), Foster SA, Klijn C, Malek S, Tissue-Specific Mutations in BRAF and EGFR Necessitate Unique Therapeutic Approaches, p. 699–701, 2016, Supplemental Reference [2] [Online Resource]). MAPK signaling pathway activation was confirmed with immunohistochemistry with anti-phospho-p44/42 MAPK [Erk1/2] [Thr202/Tyr204] (g)
