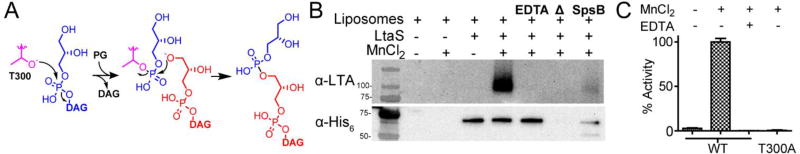
Figure 2.
LtaS activity was reconstituted in proteoliposomes. (A) Scheme for LtaS-mediated transfer of glycerol phosphate onto phosphatidyl glycerol. Glycerol phosphate from PG (blue) is loaded onto T300 (purple) of LtaS, which is then attacked by the glycerol end of another molecule of PG (red). This process repeats in a cycle in which PG is loaded onto T300 and is then offloaded onto the glycerol unit at the tip of the growing LTA polymer. (B) In vitro reconstitution of LTA biosynthesis in proteoliposomes shows that the LTA polymer is produced only when manganese is present. SpsB-catalyzed cleavage of the extracellular domain from the membrane domain abolishes LTA synthesis. (C) DAG production for WT LtaS and a mutant, T300A, containing an active site mutation. Activity is normalized to DAG produced by WT LtaS in the presence of manganese. LtaS T300A did not produce DAG or LTA polymer (Figure S10).