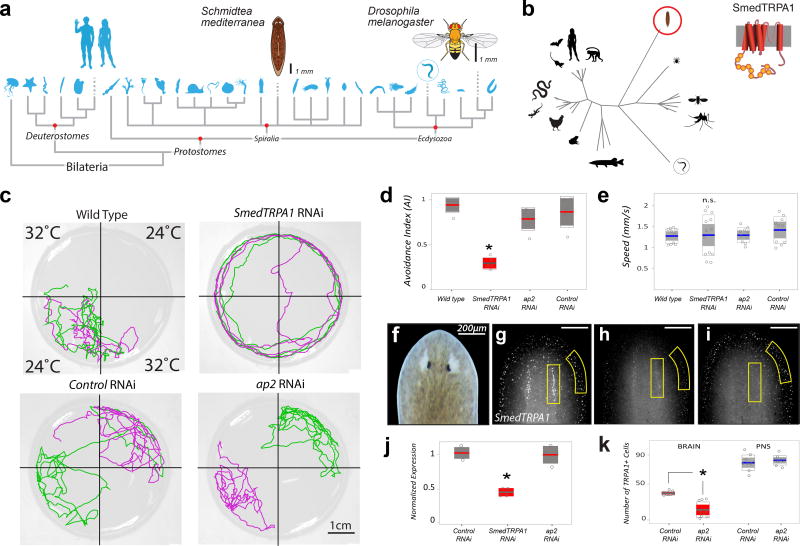
Figure 1.
Smed-TRPA1 is required for noxious heat avoidance in the planarian worm S. mediterranea. a) Phylogeny of Bilateria, showing the position of Schmidtea (C. elegans is circled). b) Phylogenetic tree constructed from an alignment of full-length TRPA1 protein sequences from a variety of species, Smed-TRPA1 is circled and a model of the channel’s structure is shown (circles=ankyrin repeats, cylinders=transmembrane domains). c) 2-choice assay for heat avoidance. In each trial two opposing floor tiles are set to 24°C and two to 32°C (noxious heat). Tracks of two worms during one such trial are shown in green and purple. Unlike wild-type, controls (unc22 RNAi), and ap2 RNAi, Smed-TRPA1 RNAi animals were not confined to the cool quadrants. d) Avoidance index for 32°C for RNAi animals. Smed-TRPA1 RNAi animals show a reduced avoidance index for heat (N= 5 groups of 10 animals, *P= 0.0054, Kruskal-Wallis; Chi-sq(3,16)=12.68). e) Smed-TRPA1 RNAi does not impact the animal’s speed of movement (N=10–13 animals; n.s. = not significantly different, P= 0.6, Kruskal-Wallis; Chi-sq(3,39)=1.48). f–i) In situ hybridization with a Smed-TRPA1 probe in (g) Control (unc22) RNAi, (h) Smed-TRPA1 RNAi and (i) ap2 RNAi animals (head region, see f), demonstrates overall reduction of mRNA by Smed-TRPA1 RNAi (independent quantification by Q-PCR is shown in j; N=4 replicates of 3 animals each, *P= 0.02, Kruskal-Wallis; Chi-sq(2,9)=7.65). k) In contrast, ap2 RNAi reduces the number of Smed-TRPA1-expressing cells in the brain region, but not in the periphery (N=9 animals, *P= 1.5 × e−5; unpaired t-test, t(16)=6.1048); in all plots, line=mean; outer boxes = +- STD; inner boxes = 95% Confidence Interval.