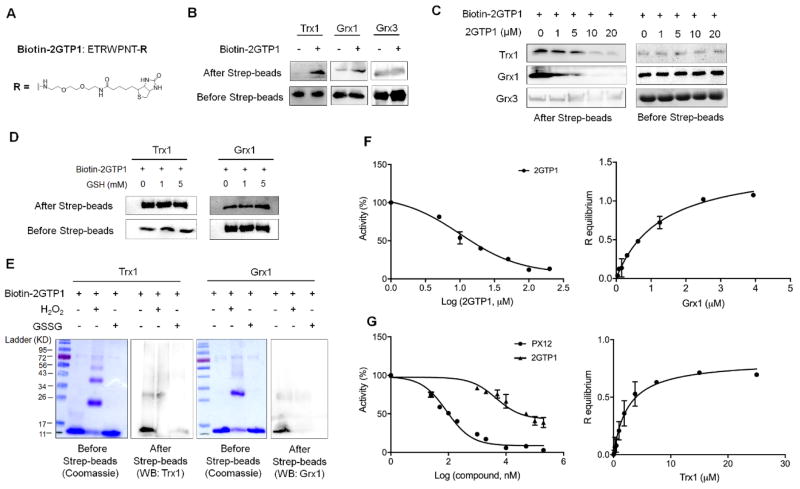
Figure 2.
In vitro binding and inhibitory analyses of 2GTP1 with Trx1 and Grx1. (A) The structure of biotinylated 2GTP1 (bitotin-2GTP1). (B–E) The binding analyses of 2GTP1 with Trx1 and Grx1. Biotin-2GTP1 was immobilized on streptavidin beads, and incubated with purified Trx1, Grx1, or Grx3 (B), which was repeated in the presence of non-biotinylated 2GTP1 as a competitor (C), reduced glutathione (D), or oxidants (10 molar equivalents to Trx1 or Grx1) (E). Trx1, Grx1, or Grx3 was analyzed by Western blotting before and after streptavidin beads. All Western blots represent data from two or three independent experiments. (F) Inhibitory activity of 2GTP1 for Grx1 deglutathionylation (left) and the binding affinity of 2GTP1 to Grx1 measured by BLI (right). (G) Inhibitory activity of 2GTP1 for Trx1-mediated insulin reduction (left) and the binding affinity of 2GTP1 to Trx1 measured by BLI (right). Data represent the mean ± SD, n= 3 independent experiments.