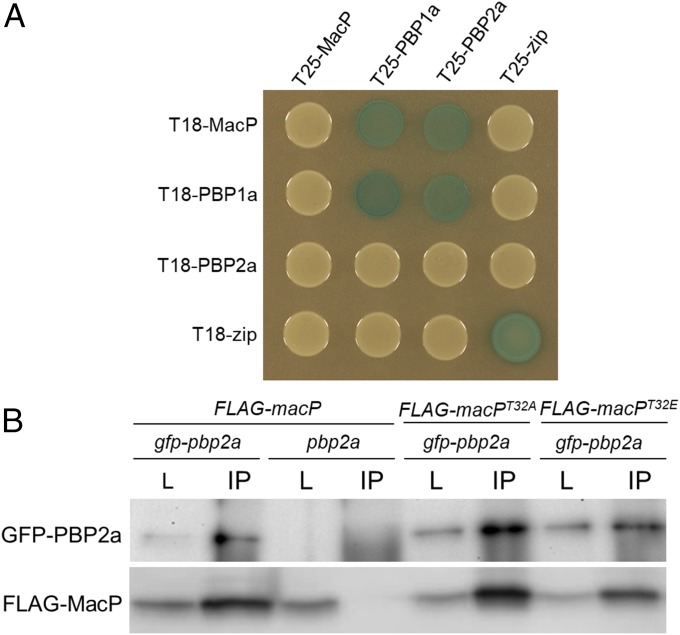
Fig. 4.
MacP interacts with both PBP2a and PBP1a. (A) BACTH interactions between MacP and aPBP enzymes. E. coli strain BTH101 (Δcya) expressing protein fusions to domains (T25 and T18) of adenylate cyclase. Strains were grown to stationary phase and 5 µL spotted on LB agar plates containing X-gal, incubated at 30 °C, and imaged. The “zip” fusions are to a leucine zipper domain and serve as both positive and negative controls. Additional controls are provided in Fig. S7. n = 3. (B) Coimmunoprecipitation of GFP–PBP2a and a functional FLAG–MacP fusion. Each fusion was expressed from the Pzn promoter using 400 µM ZnCl2. Digitonin-solubilized membrane preparations from the indicated strains were incubated with anti-GFP resin, washed, and eluted in sample buffer. Immunoblots show matched samples of solubilized membrane fractions (L) and immunoprecipitate (IP). IP samples are 20× concentrated relative to load. Fractions were probed with anti-GFP and anti-FLAG antibodies. Representative blots are shown, n = 3. Evidence of FLAG–MacP functionality, antibody specificity, additional controls, and full immunoblot analysis are provided in Fig. S8.