Figure 2. A crystal structure of human thymidine phosphorylase (TYMP).
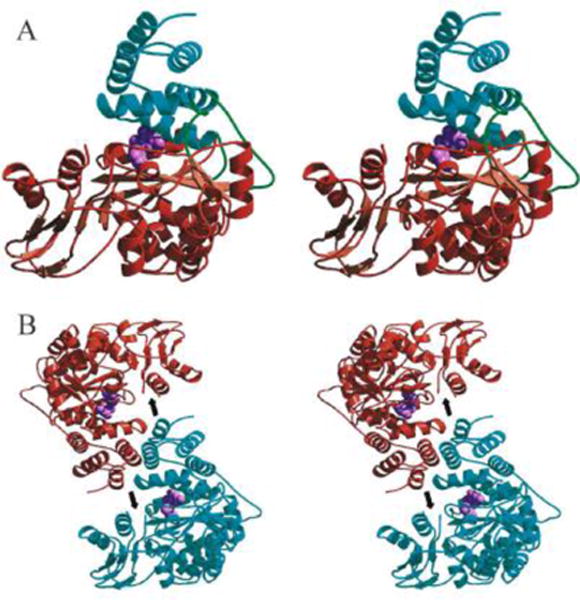
A. Stereo view of the human TYMP monomer showing the main-chain trace of the α domain (cyan), hinge regions (green), and the mixed α/β domain (brown). The TYMP inhibitor, 5-chloro-6-(1-(2-iminopyrrolidinyl)methyl) uracil (TPI; light purple) is shown as a cpk model in the active site situated between the α and α/β domains. B. Stereo view of the human TP dimer generated by applying two-fold crystallographic symmetry to the monomer. Human TYMP monomers are colored cyan and brown, and TPI (light purple) is shown as a cpk model. The position of the proteolytically cleaved loop is indicated by an arrow. (Adopted from Norman et al. Structure 2004, 12:75–85, Figure 3, with permission from Elsevier).
