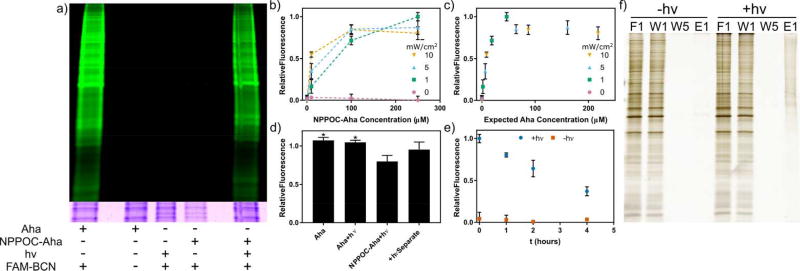
Figure 3.
In vitro analysis of light-activated bioorthogonal non-canonical amino acid tagging (laBONCAT), stability, and isolation. (a) Analysis of fluorescently labeled protein lysate by SDS-PAGE. In vitro incorporation of free L-azidohomoalanine (Aha) and Aha after photoliberation provides azide-functionalized proteins for fluorescent tagging by carboxyfluorescein-labeled bicyclononyne (FAM-BCN). Only samples incubated with free Aha or light-treated photocaged Aha (NPPOC-Aha) are fluorescently labeled. Coomassie staining indicates near-uniform protein loading. (b) The effect of light intensity and NPPOC-Aha concentration on azide incorporation into newly synthesized proteins was investigated, based on protein fluorescence. (c) The degree of incorporation from part b normalized for expected free Aha concentration. (d) Light irradiation itself does not impact ncAA incorporation of Aha (*p<0.05 by one-way ANOVA followed by Tukey’s test). (e) A significant amount of NPPOC-Aha remains stable over several hours in media and in contact with live cells suitable for labeling new proteins in tissue culture. (f) Following photomediated Aha incorporation, newly synthesized proteins can be isolated by affinity purification for downstream proteomic analysis. Fractions from left to right: flow through 1 (F1), wash 1–5 (W1–5), and elution 1 (E1).