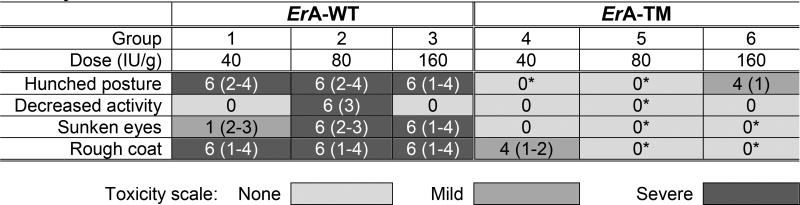
Table 3.
Acute toxicity study showing correlation between L-glutaminase activity and toxicity of the ErA variants
CD-1 mice were subjected to an acute single dose-escalation toxicity study. Each group had 3 males and 3 females, except group 6 which had only 4 animals (3 females, 1 male) due to a shortage of ErA-TM. Animals administered ErA-WT presented significant physical and behavioral symptoms, whereas the ErA-TM administered animals were largely devoid of such symptoms. The number of animals (out of 6 total) presenting symptoms is indicated. In parenthesis, the day(s) on which the symptom was observed is noted. One male in group 3 was dosed with 136 IU/g, and one female in group 5 was dosed with 60 IU/g. *p < 0.05, analysis by Fisher’s exact test: (Group 1 vs. Group 4, Group 2 vs. Group 5, Group 3 vs. Group 6). *p < 0.05, analysis by Mann-Whitney U Test: (Group 1 vs. Group 4, Group 2 vs. Group 5, Group 3 vs. Group 6). Mean with SD were plotted. See Supplementary Biostatistics on imaging for detailed standard error analysis.