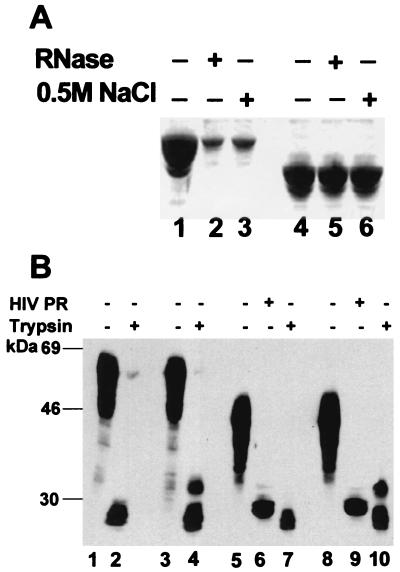
Figure 4.
Properties of particles assembled from Δ16–99 Gag protein. (A) Resistance to RNase and 0.5 M NaCl. Particles assembled from Gag (lanes 1–3) or Δ16–99 Gag (lanes 4–6) were treated with RNase (lanes 2 and 5) or 0.5 M NaCl (lanes 3 and 6). Proteins in particles pelleted after treatment were examined by SDS/PAGE and Coomassie blue staining. We estimate that >90% of the Δ16–99 Gag protein, but ≤20% of the Gag protein, remained pelletable after the treatments. (B) Susceptibility to proteolytic digestion. Particles assembled from Gag (lanes 1–4) or Δ16–99 Gag (lanes 5–10) were digested with HIV-1 PR (lanes 6 and 9) or with trypsin (lanes 2, 4, 7, and 10) after assembly in buffer B with (lanes 3–4 and 8–10) or without (lanes 1, 2, and 5–7) 2 μM IP5. Digestion products were examined as in Fig. 3.