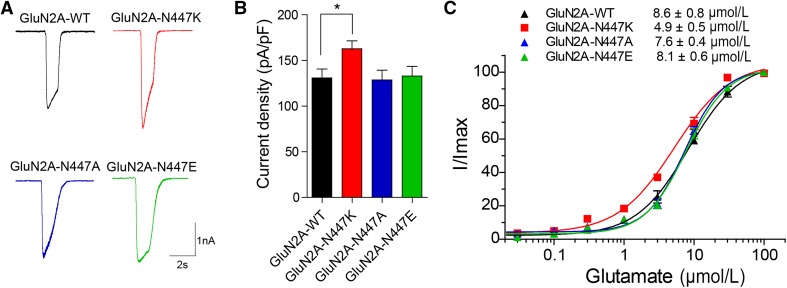
Fig. 5.
Impact of alternative substitutions at N447 on NMDAR function. A Representative current traces of GluN1/GluN2A-WT, GluN1/GluN2A-N447K, GluN1/GluN2A-N447A, and GluN1/GluN2A-N447E NMDARs evoked by 100 μmol/L glutamate and 20 μmol/L glycine at −60 mV (current scale bar, 1 nA; time scale bar, 2 s). B Quantitative analysis of whole-cell current density induced by co-agonists as in (A). C The glutamate concentration-response curves of GluN1/GluN2A-WT (black), GluN1/GluN2A-N447K (red), GluN1/GluN2A-N447A (blue), and GluN1/GluN2A-N447E (green) NMDARs were plotted by measuring the peak current evoked by graded doses of glutamate and 20 μmol/L glycine. The recordings were performed by whole-cell patch clamp at a holding potential of −60 mV after heterologous expression of the mutants and GluN1 in HEK 293T cells.