Abstract
Observations from clinical trials have frequently demonstrated that light therapy can be an effective therapy for seasonal and non-seasonal major depression. Despite the fact that light therapy is known to have several advantages over antidepressant drugs like a low cost, minimal side-effects, and fast onset of therapeutic effect, the mechanism underlying light therapy remains unclear. So far, it is known that light therapy modulates mood states and cognitive functions, involving circadian and non-circadian pathways from retinas into brain. In this review, we discuss the therapeutic effect of light on major depression and its relationship to direct retinal projections in the brain. We finally emphasize the function of the retino-raphe projection in modulating serotonin activity, which probably underlies the antidepressant effect of light therapy for depression.
Keywords: Light therapy, Depression, Retinal projection, Serotonin, Opsin
Introduction
Light, a type of energy originally from the sun, affects our mood and cognitive function [1, 2]. The retina, part of the central nervous system, has a uniquely photosensitive function [3, 4]. Light effects on mood and cognition are most likely to act through retinal circuitry and its retinofugal projections into the brain. Notably, bright light therapy appears to be a promising treatment for major depression, due to the rapid onset of benefit, minor side-effects, and low cost [5, 6]. As early as the 1980s, several clinical reports already indicated that light therapy alone is effective in treating depressive disorders [7, 8], which currently affect ~298 million people and are a leading cause of functional disability worldwide [9].
Major depression often leads to enormous personal suffering, financial costs, and an imposing social burden [10–12]. The pathophysiology of depression is still unclear but is proposed to interact with genetic and environmental factors [13]. The complexity and heterogeneity of depression have largely hampered its diagnosis and treatment [14]. Currently available antidepressants, such as selective serotonin reuptake inhibitors (SSRI), mainly exert their therapeutic effects via reducing serotonin (5-HT) turnover in the brain [15]. However, the delayed treatment effect (weeks to months) and relatively low response rate are thought to be associated with the high rate of suicide in depressed patients [14]. Other side-effects include significant weight gain and sexual dysfunction [16].
Here, we review the therapeutic effect of light therapy on depression in clinical trials and preclinical studies, and discuss the possible neural circuitry of light-signal input from retinas to brain regions, as well as their roles as potential pathways underlying light therapy for major depression.
Light Therapy for Depression in Clinical Trials
Clinical reports (Table 1) have shown, for example, that drug-free patients with major depression treated with bright light for several hours each day for a week show a significant decrease in depression ratings in comparison to patients treated with a dim red light placebo [17]. Kripke concluded that the net benefits of light therapy for major depression are similar to those resulting from antidepressant drugs including fluoxetine, sertraline, and imipramine; and the efficacy of light therapy is equal in treating seasonal and non-seasonal depression [6]. During the last three decades, however, light therapy has mainly been used to treat seasonal affective disorder (SAD) patients; this might be due to the phenomenon that light therapy seems to benefit winter depression more because of a circadian phase shift with the light stimuli [18, 19]. Emerging evidence from clinical trials using double-blind, placebo-controlled, and randomized standards has recently demonstrated that light therapy is effective in treating non-seasonal depression as well [5]. Significant efficacy of light therapy has been reported in a study using 89 elderly patients with non-seasonal major depressive disorder (MDD) when compared to randomized placebo controls [20]. Furthermore, bright light therapy as a monotherapy has been demonstrated to have a beneficial effect on 28 adolescents with non-seasonal depression [21].
Table 1.
Light therapy for seasonal and non-seasonal depression.
| Group | Light source | Wavelength | Illuminance | Time | Efficacy | References |
|---|---|---|---|---|---|---|
| 28 adolescents aged 14–17 years with mild MDD | Bright light box | White light | 2,500 lux vs 50 lux | 1 week of 2,500 lux 1 h/day | Significant differences between treatment and placebo groups | Niederhofer et al., 2012 [21] |
| 89 outpatients with MDD ≥ 60 years old, 47 randomized as control | Fluorescent tubes (Philips, HF 3304) | Pale blue in white light vs dim red light | White light at 7,500 lux vs red at 50 lux | 1 h/day, early morning, 3 weeks | Improved mood, sleep efficiency, and increased melatonin gradient | Lieverse et al., 2011[20] |
| 27 pregnant women with non-seasonal MDD, 11 randomized as control | Fluorescent bright white light | Bright white light | White light at 7,000 lux vs red light at 70 lux | 1 h/day, at home, in morning after waking, 5 weeks | Significant improvement during pregnancy, no known risk for mother and unborn child | Wirz-Justice et al., 2011 [23] |
| 18 outpatients with SAD aged 18–64 years, n = 9 each group | LED (goLITE®) | Blue light, 464 nm ± 27 nm vs blue-enriched white light, 400 nm–700 nm | Blue light at 98 lux vs white light at 711 lux | 45 min/day, in morning after waking, 3 weeks | Equal effects | Anderson et al., 2009 [132] |
| 30 individuals with SAD, 13 randomized as control | Provided by sponsor | Blue light, 470 nm vs red light, 650 nm | Blue light at 176 lux vs red light at 201 lux | 45 min daily at 06:00–08:00 am, 3 weeks | Blue light superior to red light | Strong et al., 2009 [133] |
| 26 SAD patients aged 18–65 years, 11 randomized as control | LED (Litebook) | White light with spectral emission peaks at 464 nm and 564 nm | White at 1,350 lux | 0.5 h/day, before 08:00 am, 4 weeks | Proportion of patients in remission significantly greater (SIGH-SAD < 9) | Desan et al., 2007 [134] |
| 158 patients with SAD aged 18–65 years, randomized into 6 groups | SPX-30 triphosphor fluorescent lamp (Hughes Lighting Tech) | High intensity white light vs negative air ionizer | White at 10,000 lux | 30 min/day, 10–14 days | Better response to morning than evening light | Terman et al., 1998 [18] |
| 51 drug-free in-patients with non-seasonal MDD, 26 randomized as control | Bright white light device (unknown) | Bright white light vs dim red light | White light at 2,000–3,000 lux vs dim red light | 1 week | Difference in global depression score (P = 0.02) | Kripke et al., 1992 [17] |
MDD major depressive disorder, SAD seasonal affective disorder or winter depression, LED light-emitting diode.
Although clinical cases display various remission rates gained from light therapy, mainly using different light parameters, bright light therapy has already begun to show good prospects in clinical application for many depression disorders [5, 22], including winter depression [18], antepartum depression [23], treatment-resistant depression [22], and bipolar depression [24]. Accumulating evidence has shown that light therapy is able to modulate mood state and cognitive function through circadian or non-circadian pathways [2, 25]. However, the mechanism underlying the effect of light therapy remains largely unknown [5].
Animal Studies on the Mechanism Underlying Light Therapy
In order to reveal the mechanism involved in light therapy, it has been used in animal models [5]. Iyilikci et al. reported that 1300 lux exposure to blue or white light for 10 min at zeitgebers time of 21:00 results in a significantly reduced immobility time of male Wistar rats in the second swim test relative to that of the first swim test [26]. However, that is just an acute model without any common stressors to activate a depression-like response, so the antidepressant effect was temporary but not sustained. A short photoperiod has also frequently been demonstrated to induce depression in rodents, usually that is seasonal depression [27, 28], so it was effectively rescued by light therapy [29, 30]. It has also been reported that chronic constant light (L/L cycle, 100 lux–120 lux) for 3 weeks reverses the depressive-like response in a Sprague-Dawley rat model caused by maternal separation [31]. Nevertheless, opposite results have been obtained by Nelson and colleagues using light exposure at night. They revealed that dim light (5 lux) at night running for 3–8 weeks induced depressive-like responses in nocturnal and diurnal rodents [32], including hamster [33], Nile grass rat [34], and C3H/HeNHsd mice [35]. And the aberrant light condition also disrupted normal circadian rhythms and led to impairment of mood and learning [36].
Since the therapeutic effect of light therapy is clear in the clinical trials, the animal studies have been relatively limited. Thus, more profound and convincing studies to reveal the mechanism underlying light therapy are still needed.
Neural Circuitry from Retinas to Brain Involved in Light Therapy
Key Structures of Retinal Circuitry
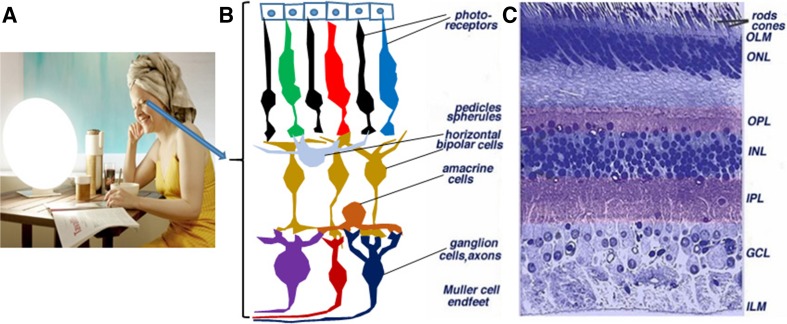
The vertebrate retina is mainly composed of three layers of neuronal somata, with two layers of synapses (Fig. 1). The outer nuclear layer contains the somata of the photoreceptors (rods and cones). The rodent retina usually contains one type of rod and two types of cone photoreceptors that are sensitive to light with short and long wavelengths, respectively. However, the primate retina has three types of cone: short, medium, and long cones. The inner nuclear layer contains the somata of the vertically-oriented bipolar cells, along with horizontal cells and amacrine cells. The ganglion cell layer harbors the somata of retinal ganglion cells (RGCs) and some displaced amacrine cells as well. These three neuronal layers are generally divided by two neuropils, in which synaptic interactions occur at crossed dendrites. The first neuropil is the outer plexiform layer where connections form between rods, cones, bipolar cells, and horizontal cells. The second neuropil is the inner plexiform layer, which functions as a relay station for carrying information vertically, mainly from the bipolar cells to the ganglion cells. In addition, amacrine cells interact in further networks to influence and integrate the signals of ganglion cells. Finally, RGCs project into the brain via their retinofugal axons [37].
Fig. 1.
Bright light therapy and the basic structure of the retina. A A lady conducting bright white light therapy in the morning (http://www.sad-lighthire.co.uk/images/gallery/256.jpg). B and C Cartoon and cross-section showing retinal structure: photoreceptors are rods and cones. The nuclei of bipolar cells are located in the INL, and retinal ganglion cells reside in the GCL, sending light signals through their axon projections into the brain. In addition, horizontal cells, amacrine cells, and Müller cells participate in the neuronal circuitry in the retina [3]. OLM, outer limiting membrane; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer; ILM, inner limiting membrane.
Retinofugal Projections from Retinas to Brain Involved in Light Therapy
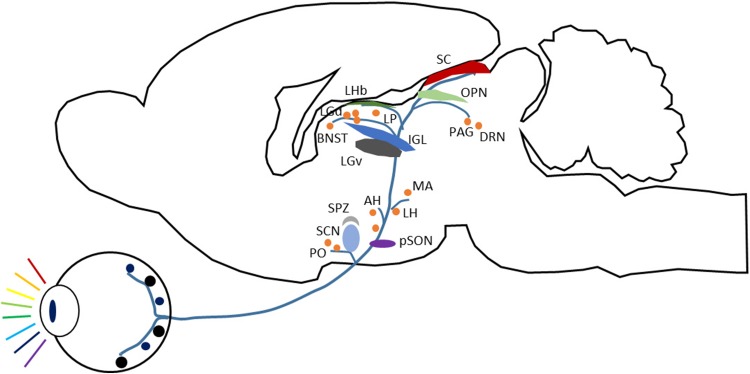
RGCs extend their axons across the inner surface of the retina in the nerve fiber layer, and then exit the eye via the optic nerve head. These axons subsequently pass along the optic nerves to the optic chiasma. They intermingle there and sort to form the two optic tracts. Finally, the axons reach their respective terminals in target nuclei [38]. In the mammalian brain (Fig. 2), ~20 nuclei receive retinal projections. For example, the lateral geniculate nucleus (LGN) is a relay to the visual cortex, the superior colliculus (SC) is responsible for visual sensory-motor processing, and the hypothalamus is mainly involved in hormone balance. Many visual target nuclei are more likely associated with mood regulation, in particular, the amygdala (Amy), bed nucleus of the stria terminalis (BNST), lateral habenula (LHb), suprachiasmatic nucleus (SCN), and dorsal raphe nucleus (DRN). Light therapy for major depression probably involves these nuclei. Some, but not all, of these nuclei are illustrated in Fig. 2.
Fig. 2.
Main retinofugal projections in the mammalian brain. Schematic of the main visual targets in the mammalian brain, based on rodent anatomy, mainly from Hattar et al., 2006 [59]. The visual targets are retinofugal but not necessarily from intrinsically photosensitive retinal ganglion cells (ipRGCs) [115]. AH, anterior hypothalamic nucleus; BNST, bed nucleus of the stria terminalis; DRN, dorsal raphe nucleus; IGL, intergeniculate leaflet; LGd, dorsal lateral geniculate nucleus; LGv, ventral lateral geniculate nucleus; LH, lateral hypothalamic area; LHb, lateral habenula, LP: lateral posterior thalamic nucleus; MA, medial amygdaloid nucleus; OPN, olivary pretectal nucleus; PAG, periaqueductal gray; PO, preoptic nucleus; pSON, peri-supraoptic nucleus; SC, superior colliculus; SCN, suprachiasmatic nucleus; SPZ, subparaventricular zone.
Suprachiasmatic Nucleus
The SCN is the principal circadian pacemaker and is located in the anterior part of the hypothalamus, above the optic chiasma [39]. As the master pacemaker for circadian rhythms, the SCN is essential for the resetting of the circadian phase by light and the photic regulation of pineal melatonin synthesis. The projection from the retinas into the SCN is dominated by intrinsically photosensitive retinal ganglion cells (ipRGCs) containing melanopsin [40]. Almost all (> 99%) of the RGCs projecting to the SCN express melanopsin in mice [40, 41], while 80%–90% of the RGCs projecting to the SCN are melanopsin-expressing ipRGCs in the rat [42] and golden hamster [43, 44]. Monocular labeling of the retinal projection is often bilaterally symmetrical in the SCN, at least in mice [45], golden hamsters [46], and macaque monkeys [47]; this differs from other main retinofugal targets such as the LGN and SC that receive more contralateral projections [48].
It is still not clear that disruption of SCN directly results in mood disorders [49], since only limited evidence shows involvement of the SCN in mood regulation [50]. And bilateral lesions of the SCN in rats have been shown to reduce depression-like behavior in the forced swim test [51]. In a postmortem study, however, an increase of arginine vasopressin (AVP) neurons along with a decrease of AVP mRNA has been reported in the SCN of depressive patients [52]. Of note, other brain regions, such as the hippocampus and LHb form many connections with the SCN [53, 54]. It is reasonable to speculate that the light effect on mood regulation may be partly due to the modulation of the neural activity in the SCN [36], though it is not the only brain region linking the light response to mood regulation [55].
Amygdala
The Amy receives relatively sparse retinal input compared with the SCN. A retinal projection into the Amy, mainly in the medial Amy, has been reported in the primate [56], hamster [57], rat [58], mouse [59, 60] and Nile grass rat [61]. The Amy is mainly implicated in the regulation of emotion, especially the formation and storage of fear memory [62–64]. Fear conditioning is a key role of the Amy, linking external stimuli to defense responses [65]. Since chronic fear induces anxiety and depression, it is reasonable that Amy dysfunction has often been associated with anxiety and depression in humans [66–68], as well as in animal models [69–71]. Using optogenetic tools, a fine connection between the basolateral Amy and the central nucleus of the Amy has been implicated as a critical neural circuit for controlling acute anxiety in mice [72], and might be involved in major depression.
Bed Nucleus of the Stria Terminalis
Retinal fiber innervation of the BNST has been observed in several species, including the primate [73], hamster [57, 74], rat [58], Spalax (blind mole rat) [75], mouse [48], and Nile grass rat [61]. The BNST in rodents and humans has been suggested to be involved in anxiety-related processes [76, 77], as well as in modulation of the hypothalamo-pituitary-adrenal (HPA) axis in response to stress [78]. The posterior BNST plays an inhibitory role in the HPA axis, while the anteroventral BNST is involved in its excitation [78]. And involvement of the HPA axis in depression is often regarded to act through hippocampal neurogenesis [79].
Animal studies on the pathophysiology of depression have focused on the BNST, but controversial results have been reported by different groups. Chemical inactivation of the BNST using cobalt chloride has been reported to have an antidepressant-like effect in rats via the forced swim test [80, 81], while bilateral electrolytic lesions of the BNST induces depression-like responses in male and female rats [82–84]. It is likely that the BNST plays bidirectional roles through its glutamatergic and GABAergic fibers to modulate stress-anxiety and depression [76, 85], respectively.
Lateral Habenula
The LHb is a key nucleus mediating communication between the forebrain and midbrain [86]. It also couples with the serotonergic system at the raphe nucleus [86, 87], and the dopaminergic system in the ventral tegmental area [88]. The LHb has been reported to contain a direct retinal projection by several groups. The first report of a retino-LHb projection was described in Spalax [75], then it was further demonstrated in the rat [89], mouse [48], and Nile grass rat [61]. According to a study by Hattar et al. [48], ipRGCs provide prominent retinal innervation of the mouse LHb.
If an unexpectedly negative outcome occurs instead of an expected reward, neurons in the LHb will be activated, sending signals into the ventral tegmental area to inhibit dopaminergic neurons [90]. It has been suggested that the LHb is involved in the stress response and major depression [91, 92]. Moreover, deep brain stimulation of the LHb has been reported to be an effective therapy for severe treatment-resistant depression [93], suggesting that the LHb is a new potential target for the treatment of depression.
Dorsal Raphe Nucleus
The DRN is the major area producing 5-HT for the forebrain, and is also one of the vital regions in response to light stimuli [55]. A retinal projection to the DRN has been found in many species, including cat [94], rat [95, 96], Mongolian gerbil [96, 97], tree shrew [98], Octodon degus [99], and the monkey Cebus apella [100]; but it is barely detectable in the mouse [48]. Light stimuli are able to significantly activate neuronal activity in the human brainstem through functional MRI detection [101], further based on the anatomical region of the human raphe [102], which suggests the existence of human retino-raphe circuitry. The 5-HT level in the human brain has been directly associated with the duration of sunlight and that can be increased rapidly with increased luminosity [103, 104]. The 5-HT output from the DRN has many functional activities, such as non-photon circadian entrainment of the pacemaker center in the SCN [105]. Although the retino-raphe projection modulates the 5-HT level [106], the action of this projection in light therapy has not yet been clearly elucidated.
Nevertheless, the DRN has frequently been highlighted in depression research [107], since the 5-HT system is thought to be an important neural circuit in depression [108, 109]. In fact, serotonergic axons from the raphe nucleus extensively innervate the cortex, hippocampus, Amy, BNST, and hypothalamus [110]. Depression seems to be more frequently associated with the DRN rather than the MRN, since reduced neuron numbers and increased mRNA levels of tryptophan hydroxylase have been reported in the DRN of depressed patients [111, 112]. When using Arvicanthis niloticus (Nile grass rat) as a model of seasonal depression with light deprivation, it was found that a projection of orexin neurons from the perifornical-lateral hypothalamic area to the DRN is an important pathway in light therapy [113]. However, the grass rat is thought to lack a direct retino-raphe projection [61]. That will differ from models with an obvious retino-raphe projection, since gerbils [114] and rats (unpublished data) both show a significant reduction of depression-like behavior by activation of the retino-raphe circuitry and by the bright light therapy; and this action is lost with specific elimination of the retino-raphe projections [114]. This finding suggests a novel neural circuit function with a retino-raphe projection underlying the effect of light therapy on depression.
In terms of retinofugal projections, the targets described above appear to be shared and have some common characteristics among various species [59, 60]. However, some targets only have sparse retinal fibers and there are some species differences, giving rise to small inconsistencies between different groups. For example, Johnson and collaborators reported that retinal projections to the BNST and Amy are absent in the rat [57]. However, the retino-raphe projection has been confirmed in the gerbil and rat by different groups [96, 115]. In addition, different regions receiving retinal projections are interconnected; for example, the 5-HT projection from the DRN strongly innervates the Amy, including its basolateral and medial areas [116–118], while the central Amy also sends afferent projections to both the dorsal and ventral DRN [119]. Thus the neural circuits can work together, contributing to the effect of light therapy on major depression.
Light Therapy Beyond Depression
Minor side-effects associated with light therapy mainly include dry eyes, sleep disturbance, and fatigue feeling; these are partly attributable to the light parameters used in therapy, such as dose (light intensity and duration of exposure), spectral content (enriched blue light or conventional white light), and method of exposure (including diffuse/focused, direct/indirect, and the angle of incidence relative to the eyes) [19]. The light parameters and conditions will be further optimized for better light therapy based on our understanding of the mechanism. However, the benefits of light therapy are likely to expand to other types of psychiatric disorders. Based on clinical case reports, these include attention deficit hyperactivity disorder [120], bipolar disorder [121], Parkinson’s disease [122], and Alzheimer’s disease [123]. Very recently, an animal study showed that the protein levels of Aβ associated with the symptoms in a mouse model of Alzheimer’s disease are significantly attenuated by LED light therapy with a gamma rhythm (40 Hz) [124]. This study implied that glial cells also take part in the effect of light therapy.
Summary and Implications
Accumulating evidence has demonstrated that light therapy is effective for seasonal and non-seasonal depression, as particularly demonstrated by double-blind, placebo-controlled, and randomized clinical trials nowadays [20, 21]. Compared with conventional treatment with antidepressant drugs like SSRI, light therapy has a relatively fast onset of therapeutic effect with minimal side-effects [19]; it might act through a unique pathway different from the chronic desensitization of pharmacological agents to 5-HT1a, 5-HT1b, and other receptors [125]. Nevertheless, it is clear that light therapy combined with psychopharmacological therapy seems to be a better option for some patients with major depression [6]. So far, the mechanism underlying the effect of light therapy is still unclear [5]. Among several emotional brain regions receiving retinal projections, the DRN with an evident retino-raphe projection appears to be a good candidate for being mainly responsible for light therapy. There is a direct retinal projection to the DRN in most rodents and primates [96, 100]. Moreover, 5-HT activity in the human brainstem occurs in response to light stimuli and is associated with depression [103, 104]. Our studies have shown that the retino-raphe projection in several species appears to be dominated by Y cells (also named alpha cells) [94, 115]. These cells with fast axonal conduction seem to match the relay condition of light signals into the 5-HT DRN system that primarily initiates the arousal response [25, 126]. Alteration of 5-HT production in the DRN is likely to underlie how light therapy exerts its therapeutic effect on depression and other mood disorders. On the other hand, current optogenetics studies have shown that light stimulation effectively modulates the activity of neurons that artificially express microbial opsins via viral transfection [127, 128]. And vertebrate opsins including rhodopsin [129], cone opsins [130], and melanopsin [131] could also be used as optogenetic tools to modulate neuronal activity. It was implicated that retinal cells with photoreceptors could affect neural circuitry function in brain, which might be a key basis for the effect of light therapy.
Acknowledgements
This review was supported by grants from the Commission on Innovation and Technology in Shenzhen Municipality of China (JCYJ20150630114942262), the Postdoctoral Science Foundation of China (2015M582440), International Postdoctoral Exchange Fellowship Program 2016 by the Office of China Postdoctoral Council (20160021), and the National Key R&D Program of China (2017YFC1310503).
Contributor Information
Xiaotao Li, Email: xtli@mit.edu.
Xiang Li, Email: xiang.li@siat.ac.cn.
References
- 1.LeGates TA, Fernandez DC, Hattar S. Light as a central modulator of circadian rhythms, sleep and affect. Nat Rev Neurosci. 2014;15:443–454. doi: 10.1038/nrn3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vandewalle G, Maquet P, Dijk DJ. Light as a modulator of cognitive brain function. Trends Cogn Sci. 2009;13:429–438. doi: 10.1016/j.tics.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Kolb H. Simple Anatomy of the Retina. In: Kolb H, Fernandez E, Nelson R, editors. Webvision: The Organization of the Retina and Visual System [Internet] Salt Lake City (UT): University of Utah Health Sciences Center; 1995. [PubMed] [Google Scholar]
- 4.Masland RH. The neuronal organization of the retina. Neuron. 2012;76:266–280. doi: 10.1016/j.neuron.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pail G, Huf W, Pjrek E, Winkler D, Willeit M, Praschak-Rieder N, et al. Bright-light therapy in the treatment of mood disorders. Neuropsychobiology. 2011;64:152–162. doi: 10.1159/000328950. [DOI] [PubMed] [Google Scholar]
- 6.Kripke D. Light treatment for nonseasonal depression: speed, efficacy, and combined treatment. J Affect Disord. 1998;49:109–117. doi: 10.1016/S0165-0327(98)00005-6. [DOI] [PubMed] [Google Scholar]
- 7.Kripke DF, Risch SC, Janowsky D. Bright white light alleviates depression. Psychiatry Res. 1983;10:105–112. doi: 10.1016/0165-1781(83)90109-9. [DOI] [PubMed] [Google Scholar]
- 8.Prasko J, Foldmann P, Praskova H, Zindr V. Hastening the onset of the effect of antidepressive agents during the use of 3 different time periods of exposure to intensive white light. Cesk Psychiatr. 1988;84:374–383. [PubMed] [Google Scholar]
- 9.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: Results from the national comorbidity survey replication (ncs-r) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 11.Lopez AD, Murray CC. The global burden of disease, 1990-2020. Nat Med. 1998;4:1241–1243. doi: 10.1038/3218. [DOI] [PubMed] [Google Scholar]
- 12.Pincus HA, Pettit AR. The societal costs of chronic major depression. J Clin Psychiatry. 2001;62(Suppl 6):5–9. [PubMed] [Google Scholar]
- 13.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duman RS, Voleti B. Signaling pathways underlying the pathophysiology and treatment of depression: novel mechanisms for rapid-acting agents. Trends Neurosci. 2012;35:47–56. doi: 10.1016/j.tins.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belmaker RH, Agam G. Major depressive disorder. N Engl J Med. 2008;358:55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- 16.Masand PS, Gupta S. Long-term side effects of newer-generation antidepressants: SSRIS, venlafaxine, nefazodone, bupropion, and mirtazapine. Ann Clin Psychiatry. 2002;14:175–182. doi: 10.3109/10401230209147454. [DOI] [PubMed] [Google Scholar]
- 17.Kripke DF, Mullaney DJ, Klauber MR, Risch SC, Gillin JC. Controlled trial of bright light for nonseasonal major depressive disorders. Biol Psychiatry. 1992;31:119–134. doi: 10.1016/0006-3223(92)90199-A. [DOI] [PubMed] [Google Scholar]
- 18.Terman M, Terman JS, Ross DC. A controlled trial of timed bright light and negative air ionization for treatment of winter depression. Arch Gen Psychiatry. 1998;55:875–882. doi: 10.1001/archpsyc.55.10.875. [DOI] [PubMed] [Google Scholar]
- 19.Terman M, Terman JS. Light therapy for seasonal and nonseasonal depression: efficacy, protocol, safety, and side effects. CNS Spectr 2005, 10: 647–663; quiz 672. [DOI] [PubMed]
- 20.Lieverse R, Van Someren EJ, Nielen MM, Uitdehaag BM, Smit JH, Hoogendijk WJ. Bright light treatment in elderly patients with nonseasonal major depressive disorder: a randomized placebo-controlled trial. Arch Gen Psychiatry. 2011;68:61–70. doi: 10.1001/archgenpsychiatry.2010.183. [DOI] [PubMed] [Google Scholar]
- 21.Niederhofer H, von Klitzing K. Bright light treatment as mono-therapy of non-seasonal depression for 28 adolescents. Int J Psychiatry Clin Pract. 2012;16:233–237. doi: 10.3109/13651501.2011.625123. [DOI] [PubMed] [Google Scholar]
- 22.Terman M. Evolving applications of light therapy. Sleep Med Rev. 2007;11:497–507. doi: 10.1016/j.smrv.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Wirz-Justice A, Bader A, Frisch U, Stieglitz RD, Alder J, Bitzer J, et al. A randomized, double-blind, placebo-controlled study of light therapy for antepartum depression. J Clin Psychiatry. 2011;72:986–993. doi: 10.4088/JCP.10m06188blu. [DOI] [PubMed] [Google Scholar]
- 24.Sit D, Wisner KL, Hanusa BH, Stull S, Terman M. Light therapy for bipolar disorder: a case series in women. Bipolar Disord. 2007;9:918–927. doi: 10.1111/j.1399-5618.2007.00451.x. [DOI] [PubMed] [Google Scholar]
- 25.Stephenson KM, Schroder CM, Bertschy G, Bourgin P. Complex interaction of circadian and non-circadian effects of light on mood: shedding new light on an old story. Sleep Med Rev. 2012;16:445–454. doi: 10.1016/j.smrv.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Iyilikci O, Aydin E, Canbeyli R. Blue but not red light stimulation in the dark has antidepressant effect in behavioral despair. Behav Brain Res. 2009;203:65–68. doi: 10.1016/j.bbr.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 27.Einat H, Kronfeld-Schor N, Eilam D. Sand rats see the light: short photoperiod induces a depression-like response in a diurnal rodent. Behav Brain Res. 2006;173:153–157. doi: 10.1016/j.bbr.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez M, Aston-Jones G. Light deprivation damages monoamine neurons and produces a depressive behavioral phenotype in rats. Proc Natl Acad Sci U S A. 2008;105:4898–4903. doi: 10.1073/pnas.0703615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krivisky K, Einat H, Kronfeld-Schor N. Effects of morning compared with evening bright light administration to ameliorate short-photoperiod induced depression- and anxiety-like behaviors in a diurnal rodent model. J Neural Transm. 2012;119:1241–1248. doi: 10.1007/s00702-012-0783-1. [DOI] [PubMed] [Google Scholar]
- 30.Ashkenazy T, Einat H, Kronfeld-Schor N. Effects of bright light treatment on depression- and anxiety-like behaviors of diurnal rodents maintained on a short daylight schedule. Behav Brain Res. 2009;201:343–346. doi: 10.1016/j.bbr.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Dimatelis JJ, Stein DJ, Russell VA. Behavioral changes after maternal separation are reversed by chronic constant light treatment. Brain Res. 2012;1480:61–71. doi: 10.1016/j.brainres.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 32.Bedrosian TA, Nelson RJ. Influence of the modern light environment on mood. Mol Psychiatry. 2013;18:751–757. doi: 10.1038/mp.2013.70. [DOI] [PubMed] [Google Scholar]
- 33.Bedrosian TA, Fonken LK, Walton JC, Haim A, Nelson RJ. Dim light at night provokes depression-like behaviors and reduces CA1 dendritic spine density in female hamsters. Psychoneuroendocrinology. 2011;36:1062–1069. doi: 10.1016/j.psyneuen.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Fonken LK, Haim A, Nelson RJ. Dim light at night increases immune function in Nile grass rats, a diurnal rodent. Chronobiol Int. 2012;29:26–34. doi: 10.3109/07420528.2011.635831. [DOI] [PubMed] [Google Scholar]
- 35.Fonken LK, Nelson RJ. Dim light at night increases depressive-like responses in male C3H/HeNHsd mice. Behav Brain Res. 2013;243:74–78. doi: 10.1016/j.bbr.2012.12.046. [DOI] [PubMed] [Google Scholar]
- 36.LeGates TA, Altimus CM, Wang H, Lee HK, Yang S, Zhao H, et al. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature. 2012;491:594–598. doi: 10.1038/nature11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodieck RW. The Vertebrate Retina: Principles of Structure and Function. San Francisco: W. H. Freeman and Company; 1973. [Google Scholar]
- 38.Berson D. Retinal ganglion cell types and their central projections. In: Basbaum AI, Kaneko A, et al. (Eds.). The Senses: A Comprehensive Reference. Academic Press, 2008, 1: 491–520.
- 39.Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Ann Rev Physiol. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pickard GE, Sollars PJ. Intrinsically photosensitive retinal ganglion cells. Rev Physiol Biochem Pharmacol. 2012;162:59–90. doi: 10.1007/112_2011_4. [DOI] [PubMed] [Google Scholar]
- 41.Baver SB, Pickard GE, Sollars PJ, Pickard GE. Two types of melanopsin retinal ganglion cell differentially innervate the hypothalamic suprachiasmatic nucleus and the olivary pretectal nucleus. Eur J Neurosci. 2008;27:1763–1770. doi: 10.1111/j.1460-9568.2008.06149.x. [DOI] [PubMed] [Google Scholar]
- 42.Gooley JJ, Lu J, Fischer D, Saper CB. A broad role for melanopsin in nonvisual photoreception. J Neurosci. 2003;23:7093–7106. doi: 10.1523/JNEUROSCI.23-18-07093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morin LP, Blanchard JH, Provencio I. Retinal ganglion cell projections to the hamster suprachiasmatic nucleus, intergeniculate leaflet, and visual midbrain: bifurcation and melanopsin immunoreactivity. J Comp Neurol. 2003;465:401–416. doi: 10.1002/cne.10881. [DOI] [PubMed] [Google Scholar]
- 44.Sollars PJ, Smeraski CA, Kaufman JD, Ogilvie MD, Provencio I, Pickard GE. Melanopsin and non-melanopsin expressing retinal ganglion cells innervate the hypothalamic suprachiasmatic nucleus. Vis Neurosci. 2003;20:601–610. doi: 10.1017/S0952523803206027. [DOI] [PubMed] [Google Scholar]
- 45.Abrahamson EE, Moore RY. Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Res. 2001;916:172–191. doi: 10.1016/S0006-8993(01)02890-6. [DOI] [PubMed] [Google Scholar]
- 46.Johnson RF, Morin LP, Moore RY. Retinohypothalamic projections in the hamster and rat demonstrated using cholera toxin. Brain Res. 1988;462:301–312. doi: 10.1016/0006-8993(88)90558-6. [DOI] [PubMed] [Google Scholar]
- 47.Hannibal J, Kankipati L, Strang CE, Peterson BB, Dacey D, Gamlin PD. Central projections of intrinsically photosensitive retinal ganglion cells in the macaque monkey. J Comp Neurol. 2014;522:2231–2248. doi: 10.1002/cne.23555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, et al. Central projections of melanopsin- expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Monteleone P, Maj M. The circadian basis of mood disorders: Recent developments and treatment implications. Eur Neuropsychopharmacol. 2008;18:701–711. doi: 10.1016/j.euroneuro.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 50.McCarthy MJ, Welsh DK. Cellular circadian clocks in mood disorders. J Biol Rhythms. 2012;27:339–352. doi: 10.1177/0748730412456367. [DOI] [PubMed] [Google Scholar]
- 51.Tataroglu O, Aksoy A, Yilmaz A, Canbeyli R. Effect of lesioning the suprachiasmatic nuclei on behavioral despair in rats. Brain Res. 2004;1001:118–124. doi: 10.1016/j.brainres.2003.11.063. [DOI] [PubMed] [Google Scholar]
- 52.Zhou JN, Riemersma RF, Unmehopa UA, Hoogendijk WJ, van Heerikhuize JJ, Hofman MA, et al. Alterations in arginine vasopressin neurons in the suprachiasmatic nucleus in depression. Arch Gen Psychiatry. 2001;58:655–662. doi: 10.1001/archpsyc.58.7.655. [DOI] [PubMed] [Google Scholar]
- 53.Wang LM, Dragich JM, Kudo T, Odom IH, Welsh DK, O’Dell TJ, et al. Expression of the circadian clock gene Period2 in the hippocampus: possible implications for synaptic plasticity and learned behaviour. ASN Neuro 2009, 1. pii: e00012. 10.1042/an20090020. [DOI] [PMC free article] [PubMed]
- 54.Zhao H, Rusak B. Circadian firing-rate rhythms and light responses of rat habenular nucleus neurons in vivo and in vitro. Neuroscience. 2005;132:519–528. doi: 10.1016/j.neuroscience.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 55.Fite KV, Wu PS, Bellemer A. Photostimulation alters c-Fos expression in the dorsal raphe nucleus. Brain Res. 2005;1031:245–252. doi: 10.1016/j.brainres.2004.10.054. [DOI] [PubMed] [Google Scholar]
- 56.Mick G, Cooper H, Magnin M. Retinal projection to the olfactory tubercle and basal telencephalon in primates. J Comp Neurol. 1993;327:205–219. doi: 10.1002/cne.903270204. [DOI] [PubMed] [Google Scholar]
- 57.Johnson RF, Morin LP, Moore RY. Retinohypothalamic projections in the hamster and rat demonstrated using cholera toxin. Brain Res. 1988;462:301–312. doi: 10.1016/0006-8993(88)90558-6. [DOI] [PubMed] [Google Scholar]
- 58.Levine JD, Weiss ML, Rosenwasser AM, Miselis RR. Retinohypothalamic tract in the female albino rat: a study using horseradish peroxidase conjugated to cholera toxin. J Comp Neurol. 1991;306:344–360. doi: 10.1002/cne.903060210. [DOI] [PubMed] [Google Scholar]
- 59.Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, et al. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morin LP, Studholme KM. Retinofugal projections in the mouse. J Comp Neurol. 2014;522:3733–3753. doi: 10.1002/cne.23635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gaillard F, Karten HJ, Sauve Y. Retinorecipient areas in the diurnal murine rodent Arvicanthis niloticus: a disproportionally large superior colliculus. J Comp Neurol. 2013;521:1699–1726. doi: 10.1002/cne.23303. [DOI] [PubMed] [Google Scholar]
- 62.Goddard GV. Functions of the amygdala. Psychol Bull. 1964;62:89–109. doi: 10.1037/h0044853. [DOI] [PubMed] [Google Scholar]
- 63.Davis M. Neurobiology of fear responses: the role of the amygdala. J Neuropsychiatry Clin Neurosci. 1997;9:382–402. doi: 10.1176/jnp.9.3.382. [DOI] [PubMed] [Google Scholar]
- 64.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 65.LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lorenzetti V, Allen NB, Whittle S, Yucel M. Amygdala volumes in a sample of current depressed and remitted depressed patients and healthy controls. J Affect Disord. 2010;120:112–119. doi: 10.1016/j.jad.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 67.Beesdo K, Lau JY, Guyer AE, McClure-Tone EB, Monk CS, Nelson EE, et al. Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Arch Gen Psychiatry. 2009;66:275–285. doi: 10.1001/archgenpsychiatry.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lau JY, Goldman D, Buzas B, Fromm SJ, Guyer AE, Hodgkinson C, et al. Amygdala function and 5-HTT gene variants in adolescent anxiety and major depressive disorder. Biol Psychiatry. 2009;65:349–355. doi: 10.1016/j.biopsych.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tasan RO, Nguyen NK, Weger S, Sartori SB, Singewald N, Heilbronn R, et al. The central and basolateral amygdala are critical sites of neuropeptide Y/Y2 receptor-mediated regulation of anxiety and depression. J Neurosci. 2010;30:6282–6290. doi: 10.1523/JNEUROSCI.0430-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mineur YS, Taylor SR, Picciotto MR. Calcineurin downregulation in the amygdala is sufficient to induce anxiety-like and depression-like behaviors in C57BL/6J male mice. Biol Psychiatry. 2014;75:991–998. doi: 10.1016/j.biopsych.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wallace TL, Stellitano KE, Neve RL, Duman RS. Effects of cyclic adenosine monophosphate response element binding protein overexpression in the basolateral amygdala on behavioral models of depression and anxiety. Biol Psychiatry. 2004;56:151–160. doi: 10.1016/j.biopsych.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 72.Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Itaya S, Van Hoesen G, Benevento L. Direct retinal pathways to the limbic thalamus of the monkey. Exp Brain Res. 1986;61:607–613. doi: 10.1007/BF00237587. [DOI] [PubMed] [Google Scholar]
- 74.Youngstrom T, Weiss M, Nunez A. Retinofugal projections to the hypothalamus, anterior thalamus and basal forebrain in hamsters. Brain Res Bull. 1991;26:403–411. doi: 10.1016/0361-9230(91)90014-B. [DOI] [PubMed] [Google Scholar]
- 75.Cooper HM, Herbin M, Nevo E. Visual system of a naturally microphthalmic mammal: the blind mole rat. Spalax ehrenbergi. J Comp Neurol. 1993;328:313–350. doi: 10.1002/cne.903280302. [DOI] [PubMed] [Google Scholar]
- 76.Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, et al. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496:224–228. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Somerville LH, Whalen PJ, Kelley WM. Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biol Psychiatry. 2010;68:416–424. doi: 10.1016/j.biopsych.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. J Neurosci. 2007;27:2025–2034. doi: 10.1523/JNEUROSCI.4301-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brown ES, Rush AJ, McEwen BS. Hippocampal remodeling and damage by corticosteroids: implications for mood disorders. Neuropsychopharmacology. 1999;21:474–484. doi: 10.1016/S0893-133X(99)00054-8. [DOI] [PubMed] [Google Scholar]
- 80.Crestani CC, Alves FH, Correa FM, Guimaraes FS, Joca SR. Acute reversible inactivation of the bed nucleus of stria terminalis induces antidepressant-like effect in the rat forced swimming test. Behav Brain Funct. 2010;6:30. doi: 10.1186/1744-9081-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nagai MM, Gomes FV, Crestani CC, Resstel LB, Joca SR. Noradrenergic neurotransmission within the bed nucleus of the stria terminalis modulates the retention of immobility in the rat forced swimming test. Behav Pharmacol. 2013;24:214–221. doi: 10.1097/FBP.0b013e3283618ae4. [DOI] [PubMed] [Google Scholar]
- 82.Pezuk P, Aydin E, Aksoy A, Canbeyli R. Effects of BNST lesions in female rats on forced swimming and navigational learning. Brain Res. 2008;1228:199–207. doi: 10.1016/j.brainres.2008.06.071. [DOI] [PubMed] [Google Scholar]
- 83.Pezuk P, Goz D, Aksoy A, Canbeyli R. BNST lesions aggravate behavioral despair but do not impair navigational learning in rats. Brain Res Bull. 2006;69:416–421. doi: 10.1016/j.brainresbull.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 84.Schulz D, Canbeyli RS. Lesion of the bed nucleus of the stria terminalis enhances learned despair. Brain Res Bull. 2000;52:83–87. doi: 10.1016/S0361-9230(00)00235-5. [DOI] [PubMed] [Google Scholar]
- 85.Sparta DR, Jennings JH, Ung RL, Stuber GD. Optogenetic strategies to investigate neural circuitry engaged by stress. Behav Brain Res. 2013;255:19–25. doi: 10.1016/j.bbr.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Herkenham M, Nauta WJ. Efferent connections of the habenular nuclei in the rat. J Comp Neurol. 1979;187:19–47. doi: 10.1002/cne.901870103. [DOI] [PubMed] [Google Scholar]
- 87.Amat J, Sparks PD, Matus-Amat P, Griggs J, Watkins LR, Maier SF. The role of the habenular complex in the elevation of dorsal raphe nucleus serotonin and the changes in the behavioral responses produced by uncontrollable stress. Brain Res. 2001;917:118–126. doi: 10.1016/S0006-8993(01)02934-1. [DOI] [PubMed] [Google Scholar]
- 88.Christoph GR, Leonzio RJ, Wilcox KS. Stimulation of the lateral habenula inhibits dopamine-containing neurons in the substantia nigra and ventral tegmental area of the rat. J Neurosci. 1986;6:613–619. doi: 10.1523/JNEUROSCI.06-03-00613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Qu T, Dong K, Sugioka K, Yamadori T. Demonstration of direct input from the retina to the lateral habenular nucleus in the albino rat. Brain Res. 1996;709:251–258. doi: 10.1016/0006-8993(95)01306-7. [DOI] [PubMed] [Google Scholar]
- 90.Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- 91.Li B, Piriz J, Mirrione M, Chung C, Proulx CD, Schulz D, et al. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature. 2011;470:535–539. doi: 10.1038/nature09742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hikosaka O. The habenula: from stress evasion to value-based decision-making. Nat Rev Neurosci. 2010;11:503–513. doi: 10.1038/nrn2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sartorius A, Kiening KL, Kirsch P, von Gall CC, Haberkorn U, Unterberg AW, et al. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol Psychiatry. 2010;67:e9–e11. doi: 10.1016/j.biopsych.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 94.Foote WE, Taber-Pierce E, Edwards L. Evidence for a retinal projection to the midbrain raphe of the cat. Brain Res. 1978;156:135–140. doi: 10.1016/0006-8993(78)90089-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shen H, Semba K. A direct retinal projection to the dorsal raphe nucleus in the rat. Brain Res. 1994;635:159–168. doi: 10.1016/0006-8993(94)91435-4. [DOI] [PubMed] [Google Scholar]
- 96.Fite KV, Janusonis S, Foote W, Bengston L. Retinal afferents to the dorsal raphe nucleus in rats and Mongolian gerbils. J Comp Neurol. 1999;414:469–484. doi: 10.1002/(SICI)1096-9861(19991129)414:4<469::AID-CNE4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 97.Luan L, Ren C, Lau BW, Yang J, Pickard GE, So KF, et al. Y-like retinal ganglion cells innervate the dorsal raphe nucleus in the Mongolian gerbil (Meriones unguiculatus) PLoS One. 2011;6:e18938. doi: 10.1371/journal.pone.0018938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Reuss S, Fuchs E. Anterograde tracing of retinal afferents to the tree shrew hypothalamus and raphe. Brain Res. 2000;874:66–74. doi: 10.1016/S0006-8993(00)02578-6. [DOI] [PubMed] [Google Scholar]
- 99.Fite KV, Janusonis S. Retinal projection to the dorsal raphe nucleus in the Chilean degus (Octodon degus) Brain Res. 2001;895:139–145. doi: 10.1016/S0006-8993(01)02061-3. [DOI] [PubMed] [Google Scholar]
- 100.Frazao R, Pinato L, da Silva AV, Britto LR, Oliveira JA, Nogueira MI. Evidence of reciprocal connections between the dorsal raphe nucleus and the retina in the monkey Cebus apella. Neurosci Lett. 2008;430:119–123. doi: 10.1016/j.neulet.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 101.Vandewalle G, Schmidt C, Albouy G, Sterpenich V, Darsaud A, Rauchs G, et al. Brain responses to violet, blue, and green monochromatic light exposures in humans: prominent role of blue light and the brainstem. PLoS One. 2007;2:e1247. doi: 10.1371/journal.pone.0001247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shibata M, Goto N, Goto J, Nonaka N. Nuclei of the human raphe. Okajimas Folia Anat Jpn. 2012;89:15–22. doi: 10.2535/ofaj.89.15. [DOI] [PubMed] [Google Scholar]
- 103.Lambert GW, Reid C, Kaye DM, Jennings GL, Esler MD. Effect of sunlight and season on serotonin turnover in the brain. Lancet. 2002;360:1840–1842. doi: 10.1016/S0140-6736(02)11737-5. [DOI] [PubMed] [Google Scholar]
- 104.Praschak-Rieder N, Willeit M, Wilson AA, Houle S, Meyer JH. Seasonal variation in human brain serotonin transporter binding. Arch Gen Psychiatry. 2008;65:1072–1078. doi: 10.1001/archpsyc.65.9.1072. [DOI] [PubMed] [Google Scholar]
- 105.Glass JD, DiNardo LA, Ehlen JC. Dorsal raphe nuclear stimulation of SCN serotonin release and circadian phase-resetting. Brain Res. 2000;859:224–232. doi: 10.1016/S0006-8993(00)01963-6. [DOI] [PubMed] [Google Scholar]
- 106.Stephenson KM, Schroder CM, Bertschy G, Bourgin P. Complex interaction of circadian and non-circadian effects of light on mood: Shedding new light on an old story. Sleep Med Rev. 2012;16:445–454. doi: 10.1016/j.smrv.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 107.Hercher C, Turecki G, Mechawar N. Through the looking glass: examining neuroanatomical evidence for cellular alterations in major depression. J Psychiatr Res. 2009;43:947–961. doi: 10.1016/j.jpsychires.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 108.Michelsen KA, Schmitz C, Steinbusch HW. The dorsal raphe nucleus—from silver stainings to a role in depression. Brain Res Rev. 2007;55:329–342. doi: 10.1016/j.brainresrev.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 109.Jasinska AJ, Lowry CA, Burmeister M. Serotonin transporter gene, stress and raphe-raphe interactions: a molecular mechanism of depression. Trends Neurosci. 2012;35:395–402. doi: 10.1016/j.tins.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 110.Tork I. Anatomy of the serotonergic system. Ann N Y Acad Sci 1990, 600: 9–34; discussion 34–35. [DOI] [PubMed]
- 111.Boldrini M, Underwood MD, Mann JJ, Arango V. More tryptophan hydroxylase in the brainstem dorsal raphe nucleus in depressed suicides. Brain Res. 2005;1041:19–28. doi: 10.1016/j.brainres.2005.01.083. [DOI] [PubMed] [Google Scholar]
- 112.Baumann B, Bielau H, Krell D, Agelink MW, Diekmann S, Wurthmann C, et al. Circumscribed numerical deficit of dorsal raphe neurons in mood disorders. Psychol Med. 2002;32:93–103. doi: 10.1017/S0033291701004822. [DOI] [PubMed] [Google Scholar]
- 113.Adidharma W, Leach G, Yan L. Orexinergic signaling mediates light-induced neuronal activation in the dorsal raphe nucleus. Neuroscience. 2012;220:201–207. doi: 10.1016/j.neuroscience.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ren C, Luan L, Wui-Man Lau B, Huang X, Yang J, Zhou Y, et al. Direct retino-raphe projection alters serotonergic tone and affective behavior. Neuropsychopharmacology. 2013;38:1163–1175. doi: 10.1038/npp.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li X, Ren C, Huang L, Lin B, Pu M, Pickard GE, et al. The dorsal raphe nucleus receives afferents from alpha-like retinal ganglion cells and intrinsically photosensitive retinal ganglion cells in the rat. Invest Ophthalmol Vis Sci. 2015;56:8373–8381. doi: 10.1167/iovs.15-16614. [DOI] [PubMed] [Google Scholar]
- 116.Steinbusch HWM. Distribution of serotonin-immunoreactivity in the central nervous system of the rat—Cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- 117.Vertes RP. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol. 1991;313:643–668. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- 118.Morin LP, Meyer-Bernstein EL. The ascending serotonergic system in the hamster: comparison with projections of the dorsal and median raphe nuclei. Neuroscience. 1999;91:81–105. doi: 10.1016/S0306-4522(98)00585-5. [DOI] [PubMed] [Google Scholar]
- 119.Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82:443–468. doi: 10.1016/S0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- 120.Van der Heijden KB, Smits MG, Van Someren EJ, Ridderinkhof KR, Gunning WB. Effect of melatonin on sleep, behavior, and cognition in ADHD and chronic sleep-onset insomnia. J Am Acad Child Adolesc Psychiatry. 2007;46:233–241. doi: 10.1097/01.chi.0000246055.76167.0d. [DOI] [PubMed] [Google Scholar]
- 121.Tseng PT, Chen YW, Tu KY, Chung W, Wang HY, Wu CK, et al. Light therapy in the treatment of patients with bipolar depression: A meta-analytic study. Eur Neuropsychopharmacol. 2016;26:1037–1047. doi: 10.1016/j.euroneuro.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 122.Willis GL, Turner EJ. Primary and secondary features of Parkinson’s disease improve with strategic exposure to bright light: a case series study. Chronobiol Int. 2007;24:521–537. doi: 10.1080/07420520701420717. [DOI] [PubMed] [Google Scholar]
- 123.Forbes D, Culum I, Lischka AR, Morgan DG, Peacock S, Forbes J, et al. Light therapy for managing cognitive, sleep, functional, behavioural, or psychiatric disturbances in dementia. Cochrane Database Syst Rev 2009: CD003946. [DOI] [PubMed]
- 124.Iaccarino HF, Singer AC, Martorell AJ, Rudenko A, Gao F, Gillingham TZ, et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature. 2016;540:230–235. doi: 10.1038/nature20587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Artigas F. Serotonin receptors involved in antidepressant effects. Pharmacol Ther. 2013;137:119–131. doi: 10.1016/j.pharmthera.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 126.Cajochen C. Alerting effects of light. Sleep Med Rev. 2007;11:453–464. doi: 10.1016/j.smrv.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 127.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 128.Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, et al. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- 129.Li X, Gutierrez DV, Hanson MG, Han J, Mark MD, Chiel H, et al. Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc Natl Acad Sci U S A. 2005;102:17816–17821. doi: 10.1073/pnas.0509030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Masseck OA, Spoida K, Dalkara D, Maejima T, Rubelowski JM, Wallhorn L, et al. Vertebrate cone opsins enable sustained and highly sensitive rapid control of Gi/o signaling in anxiety circuitry. Neuron. 2014;81:1263–1273. doi: 10.1016/j.neuron.2014.01.041. [DOI] [PubMed] [Google Scholar]
- 131.Spoida K, Masseck OA, Deneris ES, Herlitze S. Gq/5-HT2c receptor signals activate a local GABAergic inhibitory feedback circuit to modulate serotonergic firing and anxiety in mice. Proc Natl Acad Sci U S A. 2014;111:6479–6484. doi: 10.1073/pnas.1321576111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Anderson JL, Glod CA, Dai J, Cao Y, Lockley SW. Lux vs. wavelength in light treatment of Seasonal Affective Disorder. Acta Psychiatr Scand. 2009;120:203–212. doi: 10.1111/j.1600-0447.2009.01345.x. [DOI] [PubMed] [Google Scholar]
- 133.Strong RE, Marchant BK, Reimherr FW, Williams E, Soni P, Mestas R. Narrow-band blue-light treatment of seasonal affective disorder in adults and the influence of additional nonseasonal symptoms. Depress Anxiety. 2009;26:273–278. doi: 10.1002/da.20538. [DOI] [PubMed] [Google Scholar]
- 134.Desan PH, Weinstein AJ, Michalak EE, Tam EM, Meesters Y, Ruiter MJ, et al. A controlled trial of the Litebook light-emitting diode (LED) light therapy device for treatment of Seasonal Affective Disorder (SAD) BMC Psychiatry. 2007;7:38. doi: 10.1186/1471-244X-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]