Fig. 4.
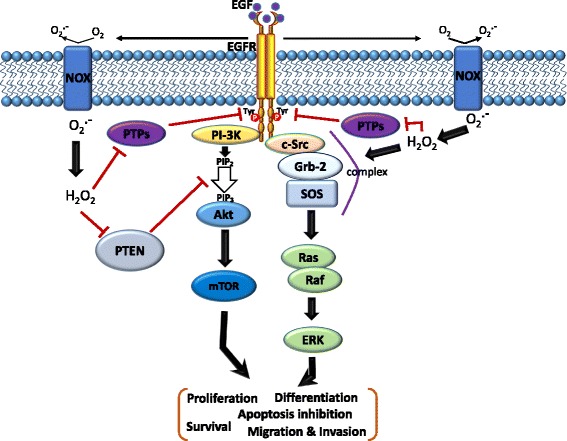
Schematic of the cross-talk between the epidermal growth factor (EGF)-EGF receptor (EGFR) axis and NADPH oxidase (NOX)-mediated reactive oxygen species (ROS) signaling pathways. The binding of EGF to the EGFR induces receptor dimerization and then autophosphorylation of tyrosine (Tyr) residues (red circles) in its cytoplasmic domain. These phosphorylated Tyr residues serve as docking sites for associated proteins which activate multiple pathways. In particular, the Ras/Raf/mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3-kinase (PI3K)/Akt pathways downstream of the EGFR play critical roles in cell migration, invasion, proliferation, and survival. Moreover, the EGF-EGFR axis also induces NOX-mediated hydrogen peroxide production, and hydrogen peroxide can diffuse across the membrane to reach the intracellular cytosol. Transient increases in hydrogen peroxide induce oxidation of reduction-oxidation reaction (redox) targets such as phosphatase and tensin homolog (PTEN) to promote Akt activation, protein tyrosine (Tyr) phosphatases (PTPs) to enhance EGFR Tyr phosphorylation, or complex formation of SHC-Grb2-SOS with the EGFR to activate Ras/MAPK signaling. Grb-2, growth factor receptor-bound protein 2; SOS, guanine nucleotide exchange protein
