Fig. 9.
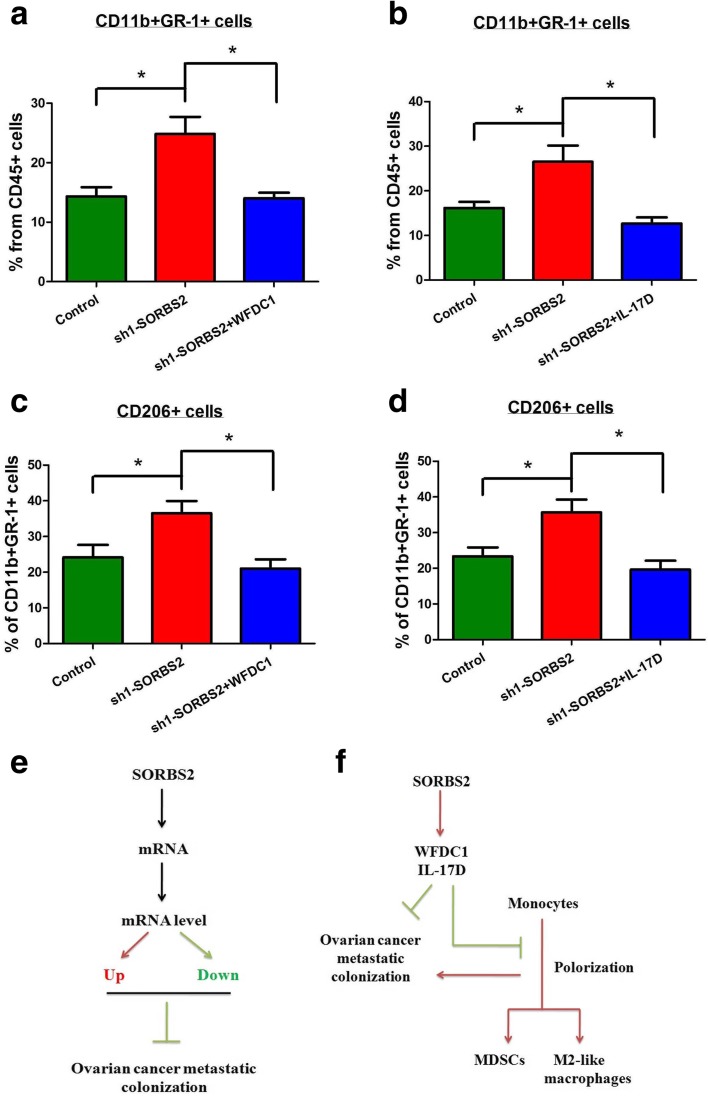
Cancer-derived SORBS2-stabilized secretome suppresses tumor metastasis and recruitment of tumor-supportive infiltrates in vivo. a The percentage of CD11b + GR-1+ cells in the CD45+ cells of the metastatic nodules of C57BL/6 mice intrabursally inoculated with control ID-8 cells, SORBS2-knockdown ID-8 cells, and WFDC1 overexpressing SORBS2-knockdown ID-8 cells. n = 6 in each group. b The percentage of CD11b + GR-1+ cells in the CD45+ cells of the metastatic nodules of C57BL/6 mice intrabursally inoculated with control ID-8 cells, SORBS2-knockdown ID-8 cells, and IL-17D-overexpressing SORBS2-knockdown ID-8 cells. n = 6 in each group. c The percentage of CD206+ cells in the CD11b + GR-1+ cells of the metastatic nodules of C57BL/6 mice intrabursally inoculated with control ID-8 cells, SORBS2-knockdown ID-8 cells, and WFDC1-overexpressing SORBS2-knockdown ID-8 cells. n = 6 in each group. d The percentage of CD206+ cells in the CD11b + GR-1+ cells of the metastatic nodules of C57BL/6 mice intrabursally inoculated with control ID-8 cells, SORBS2-knockdown ID-8 cells, and IL-17D-overexpressing SORBS2-knockdown ID-8 cells. n = 6 in each group. e At the global level, SORBS2 could bind different kinds of mRNAs and stabilize a proportion of these mRNAs. The net effect of progression-promoting and -inhibiting alterations determine whether SORBS2 loss is beneficial for ovarian cancer metastatic colonization. f At the target mRNA level, SORBS2 could stabilize the transcripts of WFDC1 and IL-17D, which leads to overexpression of these secreting factors. On one hand, they could partly suppress ovarian cancer metastasis; on the other hand, they could inhibit the polarization of monocytes towards MDSCs and M2-like macrophages, which is important for a immune suppressive tumor microenvironment favorable for ovarian cancer metastatic colonization. Data are shown as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001