Figure 3. Mrgprs mediate anaphylactic bronchoconstriction and airway hyperresponsiveness.
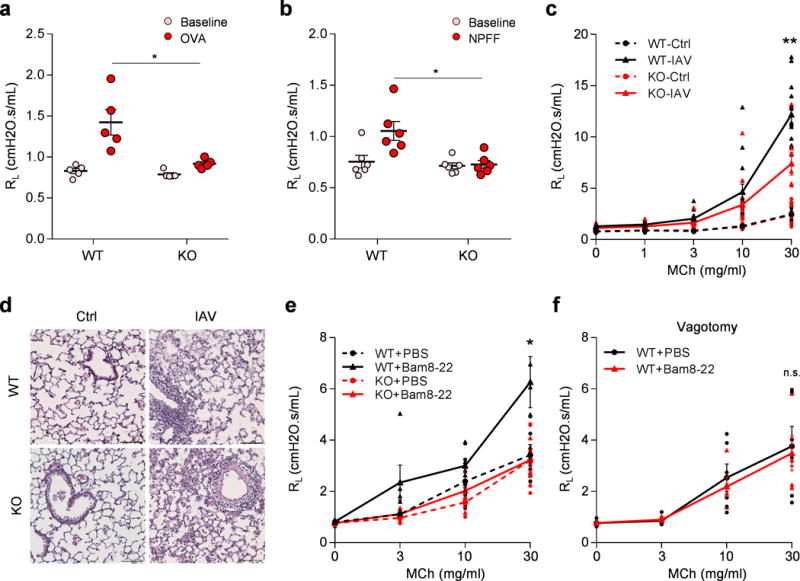
(a) Mrgpr-clusterΔ−/−mice (n=8) exhibited attenuated anaphylactic bronchoconstriction compared to WT mice (n=6). (p=0.024). (b) Retro-orbital I.V. injection of NPFF (50 μl, 10 mg/ml) induced bronchoconstriction in WT (n=6), but not Mrgpr-clusterΔ−/−mice (n=6), (p=0.014). (c) Mrgpr-clusterΔ−/− mice exhibited reduced airway hyperresponsiveness after influenza A virus inoculation. (WT-Ctrl, n=9; WT-IAV, n=15; KO-Ctrl, n=10; KO-IAV, n=12; WT-IAV vs KO-IAV, p=0.0051). (d) Representative H&E stained lung sections showing that wild-type and Mrgpr-clusterΔ−/−mice presented similar lung inflammation after influenza A virus inoculation. IFV inoculation experiment were repeated independently two times with similar results. Scale bar represents 100 μm. (e) Bam8-22 (50 μl, 10 mg/ml) enhanced the airway responsiveness to methacholine in wild-type, but not in Mrgpr-clusterΔ−/− mice. (WT+PBS, n=6; WT+Bam, n=5; KO+PBS, n=7; KO+Bam, n=5; WT+PBS vs WT+Bam, p=0.043; WT+Bam vs KO+Bam, p=0.036). (f) Vagotomy blocked Bam8-22-induced enhancement of airway responsiveness. (WT-PBS, n=6; WT-Bam, n=5; p=0.79). *p<0.05, **p< 0.01, ***p< 0.005, two-tailed unpaired Student’s t test. Data are reported as mean ± s.e.m.
