Abstract
In the management of acute ischemic stroke, vessel recanalization correlates with functional status, mortality, cost, and other outcome measures. Thrombolysis with intravenous tissue plasminogen activator has many limitations that restrict its applicability, but recent advances in the development of mechanical thrombectomy devices as well as improved systems of stroke care have resulted in greater likelihood of vessel revascularization. Nonetheless, there remains substantial discrepancy between rates of recanalization and rates of favorable outcome. The poor neurological recovery among some stroke patients despite successful recanalization confirms the need for adjuvant pharmacological therapy for neuroprotection and/or neurorestoration. Prior clinical trials of such drugs may have failed due to the inability of the agent to access the ischemic tissue beyond the occluded artery. A protocol that couples revascularization with concurrent delivery of a neuroprotectant drug offers the potential to enhance the benefit of thrombolysis. Analogs of activated protein C (APC) exert pleiotropic anti-inflammatory, anti-apoptotic, antithrombotic, cytoprotective, and neuroregenerative effects in ischemic stroke and thus appear to be promising candidates for this novel approach. A multicenter, prospective, double-blinded, dose-escalation Phase 2 randomized clinical trial has enrolled 110 patients to assess the safety, pharmacokinetics, and efficacy of human recombinant 3K3A-APC following endovascular thrombolysis.
Keywords: Activated Protein C (APC), Endovascular restorative neurosurgery, Mechanical neurothrombectomy, Neuroprotection, Neurorestoration, Stroke, Thrombolysis
Graphical abstract
1. Introduction
Stroke is the third leading cause of death worldwide and the number one cause of disability in the United States (Mortality and Causes of Death, 2015). Among the subset of stroke patients with persistent proximal vessel occlusion, up to 80% die within 90 days or fail to regain functional independence (Amar et al., 2015). It is estimated that for each minute during acute ischemic stroke (AIS), 1.9 million neurons, 14 billion synapses, and 12 km (7.5 miles) of myelinated fibers are destroyed (Saver, 2006).
Extensive research efforts have helped elucidate the pathophysiology underlying AIS and have characterized many processes of the ischemic cascade, including dysfunction of all elements of the neurovascular unit (neurons, astrocytes, microglia, and endothelial cells). Although these multiple injury mechanisms suggest myriad potential therapeutic approaches, the only medication approved by the United States Food and Drug Administration (FDA) for AIS remains intravenous tissue plasminogen activator (IV tPA), which targets the occlusive thrombus within a blood vessel. However, IV tPA has many limitations that restrict its widespread application, including a relatively short time window for delivery, low rates of recanalization in large vessel occlusion (LVO), and risks of intracranial bleeding (Amar et al., 2015). As a result of these and other reasons, only about 5% of AIS patients receive IV tPA (Jauch et al., 2013; Mozaffarian et al., 2015; Schwamm et al., 2013). Adjunctive drugs that counteract these limitations may expand the applicability of this therapy in the future.
In the last few years, the role of mechanical neurothrombectomy in AIS therapy has expanded significantly. The current generation of aspiration and stent retrieval devices achieves recanalization in the majority of patients with LVO (Almekhlafi et al., 2014; Berkhemer et al., 2015; Campbell et al., 2015; Goyal et al., 2015; Nogueira et al., 2012; Saver et al., 2015; Saver et al., 2012) and detailed analysis of safety data confirms that neurothrombectomy procedures can be performed with minimal morbidity and mortality (Akins et al., 2014).
Nonetheless, the likelihood of functional independence following neurothrombectomy (14–58%) remains poor compared with rates of recanalization (60–90%) (Amar et al., 2015). This disparity underscores the need for adjunctive therapies that enhance the benefit of endovascular thrombolysis, such as pharmacological neuroprotection (Amar et al., 2015).
Thousands of preclinical studies and human trials with potential neuroprotective agents in AIS have been reported, but none has proven unequivocally efficacious and none has yet achieved FDA approval (Ginsberg, 2009; Tymianski, 2013). One plausible explanation for this failure is that the agent may not reach the ischemic tissue due to lack of perfusion. When administered systemically, neuroprotective agents might not traverse the occluded artery and must rely instead on collateral flow to ischemic tissue, but such collateral flow may be insufficient for adequate drug delivery. This provides impetus for a strategy coupling revascularization with the ancillary administration of a neuroprotective drug.
We have previously reviewed the foundation of clinical trials that administer an analogue of activated protein C (APC) after endovascular thrombolysis by IV tPA, mechanical neurothrombectomy, or both (Amar et al., 2015). APC confers pleiotropic benefits, such as stabilizing blood brain barrier (BBB) integrity, preventing propagation of thrombosis, enhancing fibrinolysis, promoting neuroprotection, attenuating inflammation, and facilitating neuroregeneration (Griffin et al., 2002; Griffin et al., 2015; Zlokovic and Griffin, 2011). It represents a novel multiple-action multiple-target approach that addresses all components of the pathogenic triad (consisting of vascular damage, neuronal injury, and neuroinflammation) in AIS (Zlokovic and Griffin, 2011). Since the first report of its anti-inflammatory, cytoprotective, and antithrombotic properties in AIS (Shibata et al., 2001), APC has progressively fulfilled Stroke Therapy Academic Industry Roundtable (STAIR) criteria for drug development (Zlokovic and Griffin, 2011). The preclinical safety and pharmacokinetic profile of APC has been well characterized in mice and monkey models (Williams et al., 2012). A phase I safety study in normal human subjects has demonstrated that high dose bolus regimens of modified APC are well-tolerated in normal human subjects (Lyden et al., 2013), and a multicenter phase II dose-escalation clinical trial of intravenous administration for AIS (NCT02222714, NN104) is currently in progress (https://clinicaltrials.gov/ct2/show/record/NCT02222714) (Lyden et al., 2016).
2. Overview of activated protein C (APC) pathways
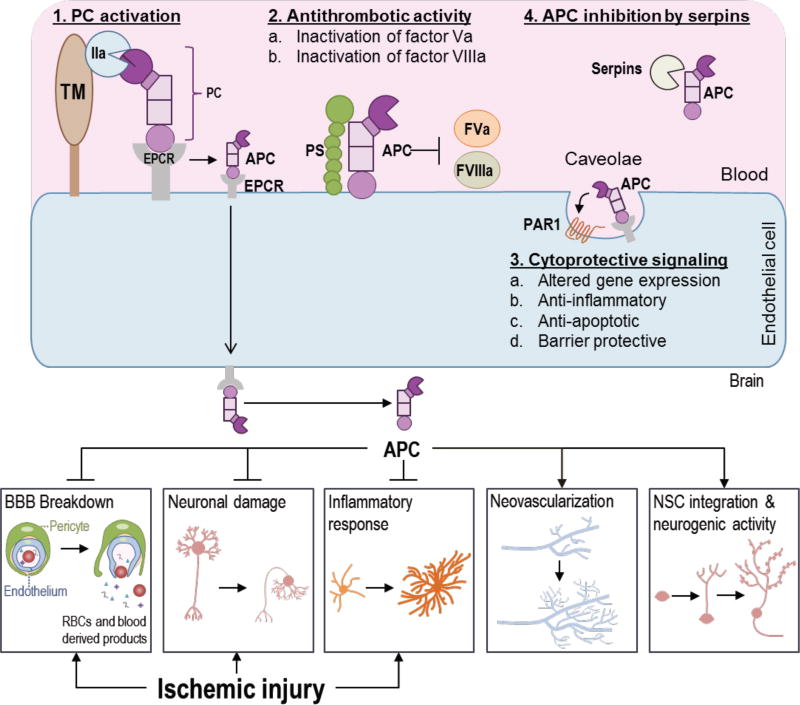
Protein C (PC) is a 62 kDa vitamin K-dependent secretary glycoprotein produced mainly by liver (Griffin et al., 1993). PC circulates at 70 nM in the blood as an inactive zymogen of the natural anticoagulant serine protease APC (Gruber and Griffin, 1992; Mosnier et al., 2007). PC binds to the endothelial cell protein C receptor (EPCR) at the endothelial cell surface (Fukudome and Esmon, 1994) and is activated by thrombomodulin (TM) receptor-bound thrombin (IIa) by cleavage at Arg169 and removal of a peptide fragment at the amino-terminal of PC heavy chain (Esmon, 2003; Essalmani et al., 2017) (Fig. 1). EPCR is also required for transport of APC across the BBB (Deane et al., 2009). APC associated with EPCR in caveolae microdomains (Russo et al., 2009) cleaves protease-activated receptor-1 (PAR-1) initiating cytoprotective signaling including altered gene expression, and anti-inflammatory, anti-apoptotic and barrier protective activities (Fig. 1). Normally plasma levels of APC are about 40 pM in healthy humans (Mosnier et al., 2007). APC is inactivated and cleared from plasma by serine protease inhibitors (Serpins) (Fig. 1). APC is a unique protease having potent anticoagulant and anti-inflammatory activities (Griffin et al., 2002). APC along with its cofactor protein S partially degrade and inactivate coagulation factors Va and VIIIa on the platelet membrane (Esmon, 2003; Marlar et al., 1982) (Fig. 1). APC is physiologically very important as heterozygous PC deficiency increases the risk for venous thrombosis in adults and the rare homozygous PC deficiency in neonates results in a fatal syndrome known as purpura fulminans if untreated (Griffin et al., 1981; Marlar et al., 1989). In mice, total deletion of PC results in death soon after birth, whereas transgenic mice expressing very low levels of PC develop severe thrombosis and inflammation (Lay et al., 2005).
Figure 1.
The Protein C (PC) pathway and the protective effects of activated protein C (APC) after ischemic injury. There are four major PC pathways. 1. PC activation. PC, bound to its receptor, endothelial protein C receptor (EPCR), is activated by thrombomodulin (TM)-bound thrombin (IIa) complex on the endothelial cell surface. 2. Antithrombotic activity. APC employs its anticoagulant activities by proteolytic inactivation of factor Va (FVa) and factor VIIIa (FVIIIa) aided by the cofactor Protein S (PS). 3. Cytoprotective signaling. APC associated with EPCR cleaves protease-activated receptor-1 (PAR-1) in caveolae initiating cytoprotective signaling including altered gene expression, and anti-inflammatory, anti-apoptotic and barrier protective activities. Other receptors (not shown) may also contribute to cytoprotective signaling. 4. APC is inhibited by serine protease inhibitors (Serpins). APC is transported across the blood-brain barrier (BBB) into brain where it has many protective and regenerative effects. APC treatment after ischemic stroke limits brain injuries by protecting the BBB and neurons and reducing inflammatory responses. Furthermore, APC promotes neovascularization and neurogenesis, and it improves neural stem cell (NSC) proliferation, integration and neurogenic activity, aiding functional recovery.
2.1. PAR1 cleavage-dependent signaling
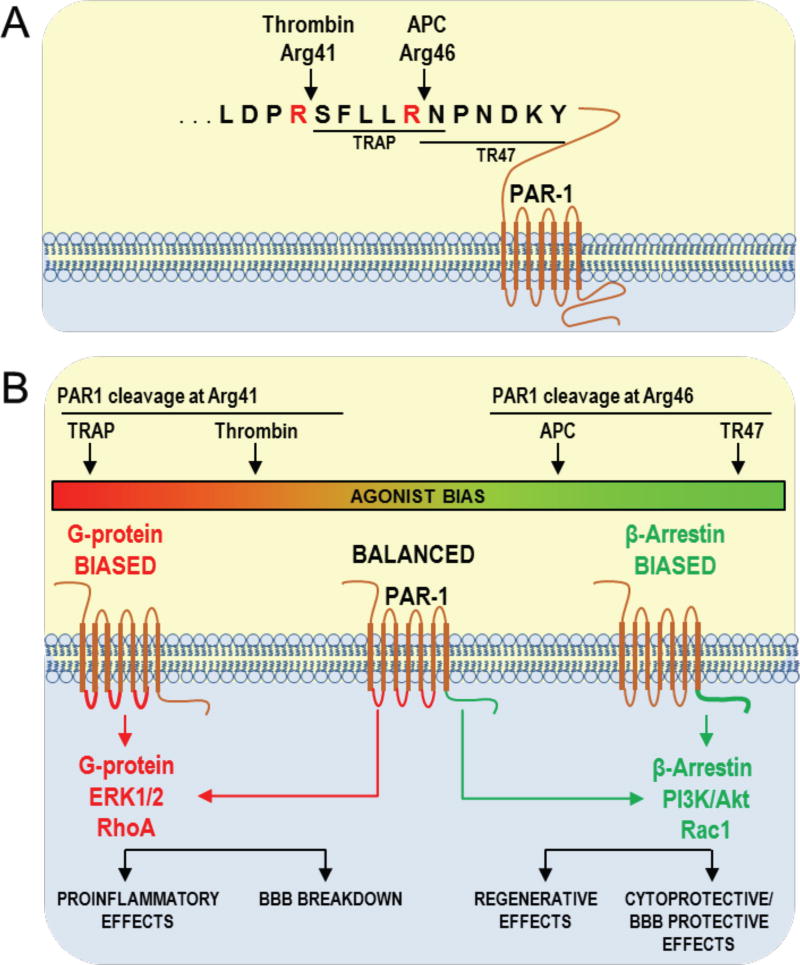
Thrombin and APC differentially cleave PAR-1 to determine and trigger different unique downstream signaling cascades (Griffin et al., 2015; Mosnier et al., 2012). Thrombin cleaves PAR1 at Arg41 generating a new N terminus beginning at Ser42 which corresponds to a PAR1 peptide sequence known as thrombin receptor-activating peptides (TRAP, peptides beginning with SFLLRN) which mirrors many of thrombin’s biological effects (Fig. 2A) (Griffin et al., 2015; Mosnier et al., 2012). APC cleaves PAR1 at Arg46 generating a new N terminus beginning with Asn47 which corresponds to a 20-mer peptide known as TR47 (i.e., a peptide starting with NPNDKY) which acts as an biased agonist of PAR1 mimicking APC’s effects (Fig. 2A) (Griffin et al., 2015; Mosnier et al., 2012). In cultured endothelial cells, TRAP or thrombin causes rapid extracellular signal-regulated kinase 1/2 (ERK 1/2) phosphorylation (Griffin et al., 2015; Mosnier et al., 2012). Conversely, TR47 or APC causes delayed protein kinase B (Akt) phosphorylation (Griffin et al., 2016). Therefore, the cleavage of PAR-1 in sites separated by only 5 amino acids has opposite biological effects depending on the biased agonism of PAR-1 (Fig. 2B) (Griffin et al., 2016; Mosnier et al., 2012). On one hand, PAR-1 can initiate G-protein-coupled receptor-dependent proinflammatory effects via ERK 1/2 activation, and Ras homolog gene family, member A (RhoA) activation, leading to BBB disruption (Fig. 2B) (Griffin et al., 2016; Griffin et al., 2015; Mosnier et al., 2012). Alternatively, activated PAR-1 can initiate a β-arrestin-2 pathway involving Ras-related C3 botulinum toxin substrate 1 (Rac1) and promoting BBB stabilization (Soh and Trejo, 2011) and survival signaling via Akt activation (Fig. 2B) (Griffin et al., 2016; Mosnier et al., 2012).
Figure 2.
PAR-1 signaling depends on the preference of thrombin or APC for different cleavage sites. A) PAR-1 is cleaved at Arg41 by thrombin resulting in an N-terminal tethered peptide. APC cleaves PAR-1 at Arg46, resulting in a different N-terminal tethered agonist. B) Thrombin cleavage of PAR-1 promotes G-protein-dependent signaling, inducing proinflammatory effects and BBB breakdown. APC cleavage of PAR-1 at Arg46 promotes β-arrestin 2-dependent signaling, inducing regenerative and cytoprotective effects. Adapted from (Mosnier et al., 2012).
2.2. The protective effects of APC
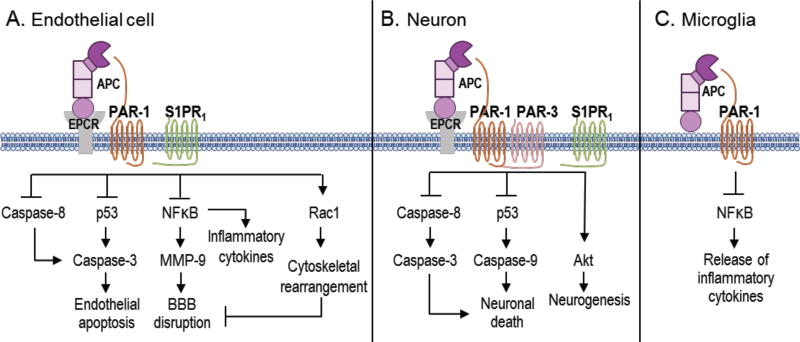
The neuroprotective effect of APC was first discovered in a murine middle cerebral artery occlusion/reperfusion model (Shibata et al., 2001). Treatment of mice with purified human plasma-derived APC reduced brain infarct volume and edema, prevented brain infiltration of neutrophils, and reduced the BBB breakdown (Shibata et al., 2001). This initial study led to the cloning of murine PC for subsequent studies to avoid cross-species artifacts (Fernandez et al., 2003). Several in vitro and in vivo preclinical studies in the past two decades have identified APC’s anti-inflammatory, cytoprotective activities which are important for direct endothelial cell protection, BBB stabilization, neuronal protection, neurogenesis and neovascularization in ischemic stroke recovery (Griffin et al., 2006; Griffin et al., 2016; Mosnier et al., 2007; Zlokovic and Griffin, 2011) (Fig. 1). Preclinical studies in various injury models showed beneficial effects of human or mouse recombinant wild-type (wt)-APC (Griffin et al., 2015). Recent clinical studies in humans demonstrated effectiveness of local application of APC in the treatment of recalcitrant orthopedic wounds (Wijewardena et al., 2011), chronic skin ulcers (Kapila et al., 2014), chronic diabetic lower leg ulcers (Whitmont et al., 2015), and pressure sores (Wijewardena et al., 2016). Treatment with recombinant wt-APC has been shown to increase the risk for bleeding (Bernard et al., 2003; Christiaans et al., 2013). To understand APC’s distinct anticoagulant and cytoprotective actions and minimize risk for intracerebral bleeding, we took advantage of APC’s unique structural features and developed several signaling-selective (with significantly reduced, <10% anticoagulant activity) and anticoagulant selective APC mutants (Griffin et al., 2015; Mosnier and Griffin, 2006). Studies with signaling-selective recombinant APC mutants, such as 5A-APC or 3K3A-APC, found significant protection in several brain injury models such as amyotrophic lateral sclerosis (Winkler et al., 2014; Zhong et al., 2009), traumatic brain injury (Petraglia et al., 2010; Walker et al., 2010) and ischemic stroke (Guo et al., 2009a; Guo et al., 2009b). Whereas treatment with anticoagulant-selective E149A-APC variant having >3-fold increased anticoagulant activity but defective cytoprotective activities worsened brain injury in ischemic stroke in mice (Wang et al., 2013a). These studies confirm that primarily, if not exclusively, the cytoprotective activities and not anticoagulant activities are central for neuroprotection after brain injury. Below, we review the literature assessing the protective effects of APC on several cell types of the neurovascular unit (NVU), including endothelial cells, neurons and microglia (Fig. 3).
Figure 3.
Cell specific APC protective signaling pathways. A) In endothelial cells, APC helps to seal the BBB and is vasculoprotective. APC/EPCR activates PAR-1 and inhibits caspase-8 activation of caspase-3, thereby limiting the extrinsic apoptotic pathway in endothelium. APC/EPCR-dependent PAR-1 activation also suppresses the pro-apoptotic p53 transcription factor inhibiting caspase-3 activation blocking the intrinsic apoptotic pathway. Also, APC suppresses the nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB)-dependent transcriptional activation of matrix metallopeptidase 9 (MMP-9), thereby blocking the breakdown of the BBB basement membrane. Furthermore, APC blocks the expression of proinflammatory cytokines limiting inflammation by controlling NFκB nuclear translocation. APCs cytoprotective effects on endothelial cells require EPCR and PAR-1 to cross-activate sphingosine 1-phosphate receptor 1 (S1PR1). Cross-activation of S1PR1 triggers Ras-related C3 botulinum toxin substrate 1 (Rac1) leading to stabilization of cytoskeleton, thereby boosting the integrity of the BBB. B) In neurons, APC/EPCR is cytoprotective via PAR-1 and PAR3 which inhibits caspase-8 upstream of caspase-3 and thereby limiting the extrinsic apoptotic pathway. Also, an APC-PAR-1-PAR3 pathway block p53 activation in injured neurons, thereby blocking the caspase-9-dependent intrinsic apoptotic pathway. Furthermore, APC promotes neurogenesis via a PAR-1-PAR3- S1PR1-Akt pathway. C) APC’s inhibition of NFκB-dependent transcriptional expression of different proinflammatory cytokines suppresses microglial activation.
2.2.1. Endothelial cells
Murine APC directly prevented apoptosis via an EPCR/PAR1-dependent pathway involving the inhibition of tumor suppressor protein p53, normalization of the apoptotic Bax/Bcl-2 ratio and reduction of caspase-3 signaling (Fig. 3A) (Cheng et al., 2003). Furthermore, APC inhibited the pro-hemorrhagic tPA-induced, NFκB-dependent matrix metalloproteinase-9 (MMP-9) pathway in ischemic brain endothelium in vivo and in vitro by acting through PAR-1 (Fig. 3A) (Cheng et al., 2006). Additionally, APC inhibited the tPA-induced caspase-8 activation of caspase-3, shifting the apoptotic signaling from the intrinsic to extrinsic pathway which requires caspase-8 (Liu et al., 2004). Interestingly, APC regulates intracellular Ca2+ levels in brain endothelial cells by binding to EPCR and signaling via PAR-1 (Domotor et al., 2003).
2.2.2. Neurons
APC inhibits caspase-3 dependent nuclear translocation of apoptosis-inducing factor in N-methyl-D-aspartate (NMDA)-treated neurons and reduced tPA-mediated cerebral ischemic injury in mice (Liu et al., 2004; Zlokovic et al., 2005). Also, APC blocked NMDA-dependent apoptosis by inhibiting caspase-8 activation upstream of caspase-3 activation and apoptosis-inducing factor nuclear translocation (Guo et al., 2004). APC also blocked the induction of p53 (Guo et al., 2004). A series of mechanistic studies by others also confirm that APC’s neuroprotection is dependent upon PAR-1, PAR-3 and EPCR (Gorbacheva et al., 2009; Gorbacheva et al., 2010; Gorbacheva et al., 2013; Gorbacheva et al., 2008; Gorbacheva et al., 2007).
2.2.3. Neurogenic effects of APC
The first evidence of APC to promote neurogenesis were in middle cerebral artery occlusion mice which found PAR-1 dependent increased proliferation of neuronal progenitor cells in the subventricular zone (SVZ) by 40–50% and migration of newly formed neuroblasts from the SVZ toward the ischemic border (Thiyagarajan et al., 2008). In vitro studies found that 3K3A-APC stimulated neuronal mitogenesis and differentiation from fetal human neural stem cells (NSCs) and neural progenitor cells (NPCs) that were mediated through PAR-1, PAR-3 and S1PR1, and triggering Akt (Guo et al., 2013). Interesting recent studies found that 3K3A-APC promotes the survival and neuronal production of transplanted NPCs into mice, leading to neuronal circuit restoration and improved function (Wang et al., 2016).
2.3. Stem cells and APC
Regenerative medicine with human stem cells holds the greatest promise for the treatment of stroke and other neurological disorders and central nervous system (CNS) injuries (George and Steinberg, 2015; Jeong et al., 2014). In the last decade, stem cell therapy has been extensively tested in preclinical experimental stroke models in rodents, large mammals and primates (Lees et al., 2012; Lemmens and Steinberg, 2013; Liu et al., 2014; Popa-Wagner et al., 2014; Shinozuka et al., 2013; Stem Cell Therapies as an Emerging Paradigm in Stroke, 2009). These studies, although they are not all consistent due to the differences in the models, cell types and transplantation protocols, have encouragingly suggested that transplanted cells homing to the damaged brain regions can exert multiple beneficial effects, including neuroprotection, anti-inflammation, pro-angiogenesis and pro-neurogenesis, and improve functional outcomes (Bliss et al., 2007; George and Steinberg, 2015; Lees et al., 2012; Liu et al., 2014). Despite recent advances, there is still a lack of mechanistic studies to address the issues regarding poor survival of transplanted cells and indiscriminate differentiation of the progenies in the hostile infarcted environment (Francis and Wei, 2010; Xian and Huang, 2015). In addition, whether transplanted cells can indeed be functional and replace the lost cells in the host neural network due to injuries has been debated. However, a recent report demonstrated that combination therapy with human NSCs and 3K3A-APC improved transplantation tolerance, stimulated cell replacement, accelerated structural recovery and enhanced functional restoration in a preclinical animal model of stroke (Wang et al., 2016). Furthermore, this study provided direct evidence showing functional integration of transplanted cells into the host neural circuits which is accompanied by substantial improvement in brain sensory-motor functions (Wang et al., 2016), suggesting that this combination approach may potentially be used for late treatment of stroke in patients.
Given that 3K3A-APC has neuroprotective effects in aged female mice and hypertensive rat models with a larger infarct volume (Wang et al., 2013b), it may be predicted that 3K3A-APC and NSC-repair therapy would successfully translate both to different experimental-stroke models and to humans (Albers et al., 2011). There are ongoing Phase I (NCT01151124) and Phase II (NCT02117635) clinical trials directly injecting manufactured NSCs into the brain of patients that remain moderately to severely disabled following an ischemic stroke. The Phase I trial, known as PISCES, found that a single intracerebral injection of up to 20 million NPCs induced no adverse events and was associated with improved neurological function (Kalladka et al., 2016). The Phase II trial recently completed recruitment and is investigating the benefit of NSC injection in ischemic stroke patients with stable upper-limb paresis. Future studies should determine whether including 3K3A-APC treatment with NPC transplantation in ongoing clinical trials could be a beneficial combination therapy for stroke patients that may help repair stroke-damaged neural circuits.
3. Augmenting the benefit of thrombolysis with APC
The premise of endovascular thrombolysis is that timely restoration of blood flow to the ischemic territory improves clinical outcome by salvaging the hypoperfused tissue at risk of converting to infarction. Meta-analysis of more than 50 studies confirms the strong correlation between recanalization and outcome in AIS, and the odds ratio of functional independence or death for those with recanalization compared to those without is 4.43 and 0.24, respectively (Rha and Saver, 2007).
However, recanalization alone –whether achieved by IV tPA, mechanical neurothrombectomy, or both—is often insufficient to achieve good clinical outcome. The current generation of neurothrombectomy devices can achieve recanalization in the overwhelming majority of patients, but even when this procedure is performed expeditiously among AIS patients with small infarct cores, the rate of good clinical outcomes is comparatively poor (Amar et al., 2015). Analysis of the reasons underlying this disparity reinforces the benefit of a strategy combining endovascular thrombolysis with APC. We have previously reviewed many of the mechanisms by which adjunctive delivery of APC should strengthen the biological relationship between recanalization and outcome (Amar et al., 2015):
Recanalization of upstream large arteries may not restore distal tissue reperfusion. Rethrombosis, migration of emboli, secondary thrombosis of downstream arteries, or microcirculatory occlusion may produce a no-reflow phenomenon despite proximal vessel thrombolysis (Bai and Lyden, 2015). The inherent anticoagulant activity of APC might attenuate the thrombosis that underlies no-reflow. Conversely, excessive anticoagulation could promote intracerebral bleeding. Variants such as 3K3A-APC, which have reduced anticoagulant activity (< 8%) compared with wild type APC, might represent a favorable compromise between prothrombotic and anticoagulant forces, but this proposition awaits confirmation in clinical trials.
Restoring flow to ischemic brain tissue following thrombolysis or thrombectomy risks reperfusion injury, hemorrhagic transformation, or cerebral edema, all of which could counteract the benefit of recanalization. By reinforcing the integrity of the damaged BBB within ischemic tissue, adjunctive APC confers vasculoprotective benefits.
It has been proposed that recanalization therapies such as tPA may be neurotoxic, either directly through induction of caspases and other proapoptotic pathways, or through breakdown of the BBB that promotes the toxic accumulation of serum proteins that affect secondary neuronal injury (del Zoppo, 1998; Liu et al., 2004; Zlokovic and Griffin, 2011). The intrinsic neuroprotective actions of adjunctive APC might overcome this damage.
Similarly, APC might protect against the deleterious effects of anesthesia, which is often administered during neurothrombectomy procedures. Unpublished data from the pooled analysis of the HERMES collaboration, reported at the 2017 International Stroke Conference, show that AIS patients given endovascular treatment under general anesthesia (GA) experienced worse neurological outcomes than those treated without GA. Among the potential explanations for this observation is the inherent neurotoxicity of intravenous and inhalational anesthetics. These actions are mediated through neuroapoptosis and inhibition of neurogenesis (Bilotta et al., 2017). The propitious effects of APC on apoptosis and neurogenesis could mitigate these effects of anesthesia on AIS patients receiving GA.
Through neuroprotection of the ischemic penumbra that has not yet converted to infarction, APC might extend the time window for the effectiveness of thrombolytic therapies. Unpublished data from the DAWN (Diffusion weighted imaging or computed tomography perfusion assessment with clinical mismatch in the triage of wake up and late presenting strokes undergoing neurointervention with Trevo) trial, presented at the 2017 European Stroke Organization Conference, indicates that neurothrombectomy is superior to standard medical therapy even when performed beyond six hours since the last known well time among the subset patients with limited infarct size and clinical-core mismatch. By sustaining the viability of the penumbral tissue through collateral flow, APC might increase the proportion of patients who can successfully undergo neurothrombectomy beyond the standard 6–8 hour time window.
If recanalization occurs too late to benefit the ischemic tissue that has progressed to infarction, the intrinsic neurogenic and angiogenic properties of APC, confirmed in both in vitro and in vivo models, might enhance functional recovery and improve clinical outcome.
These theoretical benefits of APC as an adjunct to endovascular thrombolysis form the underpinnings of the “Safety Evaluation of 3K3A-APC in Ischemic Stroke (RHAPSODY)” trial (NCT02222714, NN104). This multicenter, prospective, double-blinded, dose-escalation Phase 2 randomized clinical trial assesses the safety, pharmacokinetics, and efficacy of four increasing doses of 3K3A-APC following treatment with tPA and/or mechanical neurothrombectomy.
The RHAPSODY protocol utilizes a regimen of intravenous 3K3A-APC bolus doses every 12 hours, up to a total of 5 doses following endovascular thrombolysis. Prior studies in sepsis have shown that low-dose continuous infusion of APC is very unlikely to optimize the favorable cell signaling actions leading to cytoprotection and that bolus dosing more effectively promotes the receptor activation leading to altered gene expression profiles, which in turn contribute to the beneficial effects of APC in AIS, such a BBB stabilization and anti-apoptotic and anti-inflammatory activities (Griffin et al., 2015). The previous Phase 1 safety study in normal subjects confirmed that high dose bolus regimens using 3K3A-APC are safe and feasible in adults (Lyden et al., 2013).
The RHAPSODY trial began recruitment in 2014 and recently completed its preplanned enrollment of 110 patients, with approximately half of the study drug patients receiving IV tPA and the other half receiving thrombectomy (Lyden et al., 2016). The study results are anticipated at the end of 2017.
4. Conclusion
Adjunctive delivery of a multiple-action, multiple-target drug may augment the benefit of endovascular thrombolysis with IV tPA and/or mechanical neurothrombectomy. The anti-inflammatory, anti-apoptotic, neuroprotective, and neuroregenerative properties of APC make this agent an ideal candidate for such a strategy.
Highlights.
Stroke is a leading cause of death and disability.
Current ischemic stroke interventions have limited success rates.
3K3A-APC is a potent cytoprotective, anti-inflammatory and neuroregenerative agent.
3K3A-APC is a promising candidate for adjunctive therapy for ischemic stroke.
Acknowledgments
The authors want to acknowledge the NIH grants 9R01NS090904-16 to B.V.Z. and RO1HL052246 and PO1 HL031950 to J.H.G. for support for development of APC analogs and PAR1 mimetic peptides for stroke. Our work is also supported by Fondation Leducq Transatlantic Network of Excellence for the Study of Perivascular Spaces in Small Vessel Disease (Reference No. 16 CVD 05) to B.V.Z.
Abbreviations
- AIS
acute ischemic stroke
- Akt
protein kinase B
- APC
activated protein C
- BBB
blood-brain barrier
- CNS
central nervous system
- EPCR
endothelial protein C receptor
- ERK1/2
extracellular signal-regulated kinase 1/2
- FDA
food and drug administration
- GA
general anesthesia
- IV tPA
intravenous tissue plasminogen activator
- LVO
large vessel occlusion
- MMP9
matrix metalloproteinase-9
- NFkB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NMDA
N-methyl-D-aspartate
- NPCs
neural progenitor cells
- NSC
neural stem cell
- NVU
neurovascular unit
- PC
protein C
- PAR1
protease activated receptor 1
- PAR3
protease activated receptor 3
- Rac1
Ras-related C3 botulinum toxin substrate 1
- RhoA
Ras homolog gene family, member A
- Serpins
serine protease inhibitors
- STAIR
stroke therapy academic industry roundtable
- SVZ
subventricular zone
- IIa
thrombin
- TM
thrombomodulin
- tPA
tissue plasminogen activator
- TRAP
thrombin receptor-activating peptides
- wt
wild-type
Footnotes
Conflict of interest
Dr. Griffin is a consultant for ZZ Biotech LLC and inventor for some uses of 3K3A-APC.
Dr. Zlokovic is a founder of ZZ Biotech LLC, a biotechnology company with a mission to develop APC and its functional mutants for the treatment of stroke and other neurological disorders.
References
- Akins PT, Amar AP, Pakbaz RS, Fields JD Investigators, S. Complications of endovascular treatment for acute stroke in the SWIFT trial with solitaire and Merci devices. AJNR Am J Neuroradiol. 2014;35:524–528. doi: 10.3174/ajnr.A3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers GW, Goldstein LB, Hess DC, Wechsler LR, Furie KL, Gorelick PB, Hurn P, Liebeskind DS, Nogueira RG, Saver JL Consortium, S. V. Stroke Treatment Academic Industry Roundtable (STAIR) recommendations for maximizing the use of intravenous thrombolytics and expanding treatment options with intra-arterial and neuroprotective therapies. Stroke. 2011;42:2645–2650. doi: 10.1161/STROKEAHA.111.618850. [DOI] [PubMed] [Google Scholar]
- Almekhlafi MA, Davalos A, Bonafe A, Chapot R, Gralla J, Pereira VM, Goyal M. Impact of age and baseline NIHSS scores on clinical outcomes in the mechanical thrombectomy using solitaire FR in acute ischemic stroke study. AJNR Am J Neuroradiol. 2014;35:1337–1340. doi: 10.3174/ajnr.A3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amar AP, Griffin JH, Zlokovic BV. Combined neurothrombectomy or thrombolysis with adjunctive delivery of 3K3A-activated protein C in acute ischemic stroke. Front Cell Neurosci. 2015;9:344. doi: 10.3389/fncel.2015.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J, Lyden PD. Revisiting cerebral postischemic reperfusion injury: new insights in understanding reperfusion failure, hemorrhage, and edema. Int J Stroke. 2015;10:143–152. doi: 10.1111/ijs.12434. [DOI] [PubMed] [Google Scholar]
- Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJ, van Walderveen MA, Staals J, Hofmeijer J, van Oostayen JA, Lycklama a Nijeholt GJ, Boiten J, Brouwer PA, Emmer BJ, de Bruijn SF, van Dijk LC, Kappelle LJ, Lo RH, van Dijk EJ, de Vries J, de Kort PL, van Rooij WJ, van den Berg JS, van Hasselt BA, Aerden LA, Dallinga RJ, Visser MC, Bot JC, Vroomen PC, Eshghi O, Schreuder TH, Heijboer RJ, Keizer K, Tielbeek AV, den Hertog HM, Gerrits DG, van den Berg-Vos RM, Karas GB, Steyerberg EW, Flach HZ, Marquering HA, Sprengers ME, Jenniskens SF, Beenen LF, van den Berg R, Koudstaal PJ, van Zwam WH, Roos YB, van der Lugt A, van Oostenbrugge RJ, Majoie CB, Dippel DW. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- Bernard GR, Macias WL, Joyce DE, Williams MD, Bailey J, Vincent JL. Safety assessment of drotrecogin alfa (activated) in the treatment of adult patients with severe sepsis. Crit Care. 2003;7:155–163. doi: 10.1186/cc2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilotta F, Evered LA, Gruenbaum SE. Neurotoxicity of anesthetic drugs: an update. Curr Opin Anaesthesiol. 2017;30:452–457. doi: 10.1097/ACO.0000000000000482. [DOI] [PubMed] [Google Scholar]
- Bliss T, Guzman R, Daadi M, Steinberg GK. Cell transplantation therapy for stroke. Stroke. 2007;38:817–826. doi: 10.1161/01.STR.0000247888.25985.62. [DOI] [PubMed] [Google Scholar]
- Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ, Wu TY, Brooks M, Simpson MA, Miteff F, Levi CR, Krause M, Harrington TJ, Faulder KC, Steinfort BS, Priglinger M, Ang T, Scroop R, Barber PA, McGuinness B, Wijeratne T, Phan TG, Chong W, Chandra RV, Bladin CF, Badve M, Rice H, de Villiers L, Ma H, Desmond PM, Donnan GA, Davis SM. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- Cheng T, Liu D, Griffin JH, Fernandez JA, Castellino F, Rosen ED, Fukudome K, Zlokovic BV. Activated protein C blocks p53-mediated apoptosis in ischemic human brain endothelium and is neuroprotective. Nat Med. 2003;9:338–342. doi: 10.1038/nm826. [DOI] [PubMed] [Google Scholar]
- Cheng T, Petraglia AL, Li Z, Thiyagarajan M, Zhong Z, Wu Z, Liu D, Maggirwar SB, Deane R, Fernandez JA, LaRue B, Griffin JH, Chopp M, Zlokovic BV. Activated protein C inhibits tissue plasminogen activator-induced brain hemorrhage. Nat Med. 2006;12:1278–1285. doi: 10.1038/nm1498. [DOI] [PubMed] [Google Scholar]
- Christiaans SC, Wagener BM, Esmon CT, Pittet JF. Protein C and acute inflammation: a clinical and biological perspective. Am J Physiol Lung Cell Mol Physiol. 2013;305:L455–466. doi: 10.1152/ajplung.00093.2013. [DOI] [PubMed] [Google Scholar]
- Deane R, LaRue B, Sagare AP, Castellino FJ, Zhong Z, Zlokovic BV. Endothelial protein C receptor-assisted transport of activated protein C across the mouse blood-brain barrier. J Cereb Blood Flow Metab. 2009;29:25–33. doi: 10.1038/jcbfm.2008.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Zoppo GJ. tPA: a neuron buster, too? Nat Med. 1998;4:148–150. doi: 10.1038/nm0298-148. [DOI] [PubMed] [Google Scholar]
- Domotor E, Benzakour O, Griffin JH, Yule D, Fukudome K, Zlokovic BV. Activated protein C alters cytosolic calcium flux in human brain endothelium via binding to endothelial protein C receptor and activation of protease activated receptor-1. Blood. 2003;101:4797–4801. doi: 10.1182/blood-2002-12-3680. [DOI] [PubMed] [Google Scholar]
- Esmon CT. The protein C pathway. Chest. 2003;124:26S–32S. doi: 10.1378/chest.124.3_suppl.26s. [DOI] [PubMed] [Google Scholar]
- Essalmani R, Susan-Resiga D, Guillemot J, Kim W, Sachan V, Awan Z, Chamberland A, Asselin MC, Ly K, Desjardins R, Day R, Prat A, Seidah NG. Thrombin activation of protein C requires prior processing by a liver proprotein convertase. J Biol Chem. 2017;292:10564–10573. doi: 10.1074/jbc.M116.770040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez JA, Xu X, Liu D, Zlokovic BV, Griffin JH. Recombinant murine-activated protein C is neuroprotective in a murine ischemic stroke model. Blood Cells Mol Dis. 2003;30:271–276. doi: 10.1016/s1079-9796(03)00034-2. [DOI] [PubMed] [Google Scholar]
- Francis KR, Wei L. Human embryonic stem cell neural differentiation and enhanced cell survival promoted by hypoxic preconditioning. Cell Death Dis. 2010;1:e22. doi: 10.1038/cddis.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukudome K, Esmon CT. Identification, cloning, and regulation of a novel endothelial cell protein C/activated protein C receptor. J Biol Chem. 1994;269:26486–26491. [PubMed] [Google Scholar]
- George PM, Steinberg GK. Novel Stroke Therapeutics: Unraveling Stroke Pathophysiology and Its Impact on Clinical Treatments. Neuron. 2015;87:297–309. doi: 10.1016/j.neuron.2015.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg MD. Current status of neuroprotection for cerebral ischemia: synoptic overview. Stroke. 2009;40:S111–114. doi: 10.1161/STROKEAHA.108.528877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbacheva L, Davidova O, Sokolova E, Ishiwata S, Pinelis V, Strukova S, Reiser G. Endothelial protein C receptor is expressed in rat cortical and hippocampal neurons and is necessary for protective effect of activated protein C at glutamate excitotoxicity. J Neurochem. 2009;111:967–975. doi: 10.1111/j.1471-4159.2009.06380.x. [DOI] [PubMed] [Google Scholar]
- Gorbacheva L, Pinelis V, Ishiwata S, Strukova S, Reiser G. Activated protein C prevents glutamate- and thrombin-induced activation of nuclear factor-kappaB in cultured hippocampal neurons. Neuroscience. 2010;165:1138–1146. doi: 10.1016/j.neuroscience.2009.11.027. [DOI] [PubMed] [Google Scholar]
- Gorbacheva L, Strukova S, Pinelis V, Ishiwata S, Stricker R, Reiser G. NF-kappaB-dependent and -independent pathways in the protective effects of activated protein C in hippocampal and cortical neurons at excitotoxicity. Neurochem Int. 2013;63:101–111. doi: 10.1016/j.neuint.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Gorbacheva LR, Storozhevykh TP, Pinelis VG, Davydova ON, Ishiwata S, Strukova SM. Activated protein C via PAR1 receptor regulates survival of neurons under conditions of glutamate excitotoxicity. Biochemistry (Mosc) 2008;73:717–724. doi: 10.1134/s0006297908060138. [DOI] [PubMed] [Google Scholar]
- Gorbacheva LR, Storozhevykh TP, Pinelis VG, Ishiwata S, Strukova SM. Protease-activated receptor (PAR)1-mediated anti-apoptotic effect of activated protein C on glutamate excitotoxicity in hippocampal neurons. Thromb Haemost. 2007;98:1150–1152. doi: 10.1160/th07-03-0228. [DOI] [PubMed] [Google Scholar]
- Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL, Dowlatshahi D, Frei DF, Kamal NR, Montanera WJ, Poppe AY, Ryckborst KJ, Silver FL, Shuaib A, Tampieri D, Williams D, Bang OY, Baxter BW, Burns PA, Choe H, Heo JH, Holmstedt CA, Jankowitz B, Kelly M, Linares G, Mandzia JL, Shankar J, Sohn SI, Swartz RH, Barber PA, Coutts SB, Smith EE, Morrish WF, Weill A, Subramaniam S, Mitha AP, Wong JH, Lowerison MW, Sajobi TT, Hill MD. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–1030. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- Griffin JH, Evatt B, Wideman C, Fernandez JA. Anticoagulant protein C pathway defective in majority of thrombophilic patients. Blood. 1993;82:1989–1993. [PubMed] [Google Scholar]
- Griffin JH, Evatt B, Zimmerman TS, Kleiss AJ, Wideman C. Deficiency of protein C in congenital thrombotic disease. J Clin Invest. 1981;68:1370–1373. doi: 10.1172/JCI110385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JH, Fernandez JA, Mosnier LO, Liu D, Cheng T, Guo H, Zlokovic BV. The promise of protein C. Blood Cells Mol Dis. 2006;36:211–216. doi: 10.1016/j.bcmd.2005.12.023. [DOI] [PubMed] [Google Scholar]
- Griffin JH, Mosnier LO, Fernandez JA, Zlokovic BV. 2016 Scientific Sessions Sol Sherry Distinguished Lecturer in Thrombosis: Thrombotic Stroke: Neuroprotective Therapy by Recombinant-Activated Protein C. Arterioscler Thromb Vasc Biol. 2016;36:2143–2151. doi: 10.1161/ATVBAHA.116.308038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JH, Zlokovic B, Fernandez JA. Activated protein C: potential therapy for severe sepsis, thrombosis, and stroke. Semin Hematol. 2002;39:197–205. doi: 10.1053/shem.2002.34093. [DOI] [PubMed] [Google Scholar]
- Griffin JH, Zlokovic BV, Mosnier LO. Activated protein C: biased for translation. Blood. 2015;125:2898–2907. doi: 10.1182/blood-2015-02-355974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber A, Griffin JH. Direct detection of activated protein C in blood from human subjects. Blood. 1992;79:2340–2348. [PubMed] [Google Scholar]
- Guo H, Liu D, Gelbard H, Cheng T, Insalaco R, Fernandez JA, Griffin JH, Zlokovic BV. Activated protein C prevents neuronal apoptosis via protease activated receptors 1 and 3. Neuron. 2004;41:563–572. doi: 10.1016/s0896-6273(04)00019-4. [DOI] [PubMed] [Google Scholar]
- Guo H, Singh I, Wang Y, Deane R, Barrett T, Fernandez JA, Chow N, Griffin JH, Zlokovic BV. Neuroprotective activities of activated protein C mutant with reduced anticoagulant activity. Eur J Neurosci. 2009a;29:1119–1130. doi: 10.1111/j.1460-9568.2009.06664.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Guo H, Wang Y, Singh I, Liu D, Fernandez JA, Griffin JH, Chow N, Zlokovic BV. Species-dependent neuroprotection by activated protein C mutants with reduced anticoagulant activity. J Neurochem. 2009b;109:116–124. doi: 10.1111/j.1471-4159.2009.05921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Zhao Z, Yang Q, Wang M, Bell RD, Wang S, Chow N, Davis TP, Griffin JH, Goldman SA, Zlokovic BV. An activated protein C analog stimulates neuronal production by human neural progenitor cells via a PAR1-PAR3-S1PR1-Akt pathway. J Neurosci. 2013;33:6181–6190. doi: 10.1523/JNEUROSCI.4491-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauch EC, Saver JL, Adams HP, Jr, Bruno A, Connors JJ, Demaerschalk BM, Khatri P, McMullan PW, Jr, Qureshi AI, Rosenfield K, Scott PA, Summers DR, Wang DZ, Wintermark M, Yonas H American Heart Association Stroke, C., Council on Cardiovascular, N., Council on Peripheral Vascular, D., Council on Clinical, C. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- Jeong H, Yim HW, Cho YS, Kim YI, Jeong SN, Kim HB, Oh IH. Efficacy and safety of stem cell therapies for patients with stroke: a systematic review and single arm meta-analysis. Int J Stem Cells. 2014;7:63–69. doi: 10.15283/ijsc.2014.7.2.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalladka D, Sinden J, Pollock K, Haig C, McLean J, Smith W, McConnachie A, Santosh C, Bath PM, Dunn L, Muir KW. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): a phase 1, first-in-man study. Lancet. 2016;388:787–796. doi: 10.1016/S0140-6736(16)30513-X. [DOI] [PubMed] [Google Scholar]
- Kapila S, Reid I, Dixit S, Fulcher G, March L, Jackson C, Cooper A. Use of dermal injection of activated protein C for treatment of large chronic wounds secondary to pyoderma gangrenosum. Clin Exp Dermatol. 2014;39:785–790. doi: 10.1111/ced.12361. [DOI] [PubMed] [Google Scholar]
- Lay AJ, Liang Z, Rosen ED, Castellino FJ. Mice with a severe deficiency in protein C display prothrombotic and proinflammatory phenotypes and compromised maternal reproductive capabilities. J Clin Invest. 2005;115:1552–1561. doi: 10.1172/JCI24030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees JS, Sena ES, Egan KJ, Antonic A, Koblar SA, Howells DW, Macleod MR. Stem cell-based therapy for experimental stroke: a systematic review and meta-analysis. Int J Stroke. 2012;7:582–588. doi: 10.1111/j.1747-4949.2012.00797.x. [DOI] [PubMed] [Google Scholar]
- Lemmens R, Steinberg GK. Stem cell therapy for acute cerebral injury: what do we know and what will the future bring? Curr Opin Neurol. 2013;26:617–625. doi: 10.1097/WCO.0000000000000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Cheng T, Guo H, Fernandez JA, Griffin JH, Song X, Zlokovic BV. Tissue plasminogen activator neurovascular toxicity is controlled by activated protein C. Nat Med. 2004;10:1379–1383. doi: 10.1038/nm1122. [DOI] [PubMed] [Google Scholar]
- Liu X, Ye R, Yan T, Yu SP, Wei L, Xu G, Fan X, Jiang Y, Stetler RA, Liu G, Chen J. Cell based therapies for ischemic stroke: from basic science to bedside. Prog Neurobiol. 2014;115:92–115. doi: 10.1016/j.pneurobio.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyden P, Levy H, Weymer S, Pryor K, Kramer W, Griffin JH, Davis TP, Zlokovic B. Phase 1 safety, tolerability and pharmacokinetics of 3K3A-APC in healthy adult volunteers. Curr Pharm Des. 2013;19:7479–7485. doi: 10.2174/1381612819666131230131454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyden P, Weymer S, Coffey C, Cudkowicz M, Berg S, O'Brien S, Fisher M, Haley EC, Khatri P, Saver J, Levine S, Levy H, Rymer M, Wechsler L, Jadhav A, McNeil E, Waddy S, Pryor K. Selecting Patients for Intra-Arterial Therapy in the Context of a Clinical Trial for Neuroprotection. Stroke. 2016;47:2979–2985. doi: 10.1161/STROKEAHA.116.013881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlar RA, Kleiss AJ, Griffin JH. Mechanism of action of human activated protein C, a thrombin-dependent anticoagulant enzyme. Blood. 1982;59:1067–1072. [PubMed] [Google Scholar]
- Marlar RA, Montgomery RR, Broekmans AW. Diagnosis and treatment of homozygous protein C deficiency. Report of the Working Party on Homozygous Protein C Deficiency of the Subcommittee on Protein C and Protein S, International Committee on Thrombosis and Haemostasis. J Pediatr. 1989;114:528–534. doi: 10.1016/s0022-3476(89)80688-2. [DOI] [PubMed] [Google Scholar]
- Mortality GBD Causes of Death, C. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosnier LO, Griffin JH. Protein C anticoagulant activity in relation to anti-inflammatory and anti-apoptotic activities. Front Biosci. 2006;11:2381–2399. doi: 10.2741/1977. [DOI] [PubMed] [Google Scholar]
- Mosnier LO, Sinha RK, Burnier L, Bouwens EA, Griffin JH. Biased agonism of protease-activated receptor 1 by activated protein C caused by noncanonical cleavage at Arg46. Blood. 2012;120:5237–5246. doi: 10.1182/blood-2012-08-452169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosnier LO, Zlokovic BV, Griffin JH. The cytoprotective protein C pathway. Blood. 2007;109:3161–3172. doi: 10.1182/blood-2006-09-003004. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB American Heart Association Statistics, C., Stroke Statistics, S. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- Nogueira RG, Lutsep HL, Gupta R, Jovin TG, Albers GW, Walker GA, Liebeskind DS, Smith WS. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet. 2012;380:1231–1240. doi: 10.1016/S0140-6736(12)61299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petraglia AL, Marky AH, Walker C, Thiyagarajan M, Zlokovic BV. Activated protein C is neuroprotective and mediates new blood vessel formation and neurogenesis after controlled cortical impact. Neurosurgery. 2010;66:165–171. doi: 10.1227/01.NEU.0000363148.49779.68. discussion 171-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa-Wagner A, Buga AM, Doeppner TR, Hermann DM. Stem cell therapies in preclinical models of stroke associated with aging. Front Cell Neurosci. 2014;8:347. doi: 10.3389/fncel.2014.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007;38:967–973. doi: 10.1161/01.STR.0000258112.14918.24. [DOI] [PubMed] [Google Scholar]
- Russo A, Soh UJ, Paing MM, Arora P, Trejo J. Caveolae are required for protease-selective signaling by protease-activated receptor-1. Proc Natl Acad Sci U S A. 2009;106:6393–6397. doi: 10.1073/pnas.0810687106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saver JL. Time is brain--quantified. Stroke. 2006;37:263–266. doi: 10.1161/01.STR.0000196957.55928.ab. [DOI] [PubMed] [Google Scholar]
- Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, Albers GW, Cognard C, Cohen DJ, Hacke W, Jansen O, Jovin TG, Mattle HP, Nogueira RG, Siddiqui AH, Yavagal DR, Baxter BW, Devlin TG, Lopes DK, Reddy VK, de Rochemont RD, Singer OC, Jahan R. Stent-Retriever Thrombectomy after Intravenous t-PA vs. t-PA Alone in Stroke. N Engl J Med. 2015 doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- Saver JL, Jahan R, Levy EI, Jovin TG, Baxter B, Nogueira RG, Clark W, Budzik R, Zaidat OO. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012;380:1241–1249. doi: 10.1016/S0140-6736(12)61384-1. [DOI] [PubMed] [Google Scholar]
- Schwamm LH, Ali SF, Reeves MJ, Smith EE, Saver JL, Messe S, Bhatt DL, Grau-Sepulveda MV, Peterson ED, Fonarow GC. Temporal trends in patient characteristics and treatment with intravenous thrombolysis among acute ischemic stroke patients at Get With The Guidelines-Stroke hospitals. Circ Cardiovasc Qual Outcomes. 2013;6:543–549. doi: 10.1161/CIRCOUTCOMES.111.000303. [DOI] [PubMed] [Google Scholar]
- Shibata M, Kumar SR, Amar A, Fernandez JA, Hofman F, Griffin JH, Zlokovic BV. Anti-inflammatory, antithrombotic, and neuroprotective effects of activated protein C in a murine model of focal ischemic stroke. Circulation. 2001;103:1799–1805. doi: 10.1161/01.cir.103.13.1799. [DOI] [PubMed] [Google Scholar]
- Shinozuka K, Dailey T, Tajiri N, Ishikawa H, Kaneko Y, Borlongan CV. Stem cell transplantation for neuroprotection in stroke. Brain Sci. 2013;3:239–261. doi: 10.3390/brainsci3010239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soh UJ, Trejo J. Activated protein C promotes protease-activated receptor-1 cytoprotective signaling through beta-arrestin and dishevelled-2 scaffolds. Proc Natl Acad Sci U S A. 2011;108:E1372–1380. doi: 10.1073/pnas.1112482108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stem Cell Therapies as an Emerging Paradigm in Stroke, P. Stem Cell Therapies as an Emerging Paradigm in Stroke (STEPS): bridging basic and clinical science for cellular and neurogenic factor therapy in treating stroke. Stroke. 2009;40:510–515. doi: 10.1161/STROKEAHA.108.526863. [DOI] [PubMed] [Google Scholar]
- Thiyagarajan M, Fernandez JA, Lane SM, Griffin JH, Zlokovic BV. Activated protein C promotes neovascularization and neurogenesis in postischemic brain via protease-activated receptor 1. J Neurosci. 2008;28:12788–12797. doi: 10.1523/JNEUROSCI.3485-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tymianski M. Novel approaches to neuroprotection trials in acute ischemic stroke. Stroke. 2013;44:2942–2950. doi: 10.1161/STROKEAHA.113.000731. [DOI] [PubMed] [Google Scholar]
- Walker CT, Marky AH, Petraglia AL, Ali T, Chow N, Zlokovic BV. Activated protein C analog with reduced anticoagulant activity improves functional recovery and reduces bleeding risk following controlled cortical impact. Brain Res. 2010;1347:125–131. doi: 10.1016/j.brainres.2010.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Sinha RK, Mosnier LO, Griffin JH, Zlokovic BV. Neurotoxicity of the anticoagulant-selective E149A-activated protein C variant after focal ischemic stroke in mice. Blood Cells Mol Dis. 2013a;51:104–108. doi: 10.1016/j.bcmd.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhao Z, Chow N, Rajput PS, Griffin JH, Lyden PD, Zlokovic BV. Activated protein C analog protects from ischemic stroke and extends the therapeutic window of tissue-type plasminogen activator in aged female mice and hypertensive rats. Stroke. 2013b;44:3529–3536. doi: 10.1161/STROKEAHA.113.003350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhao Z, Rege SV, Wang M, Si G, Zhou Y, Wang S, Griffin JH, Goldman SA, Zlokovic BV. 3K3A-activated protein C stimulates postischemic neuronal repair by human neural stem cells in mice. Nat Med. 2016;22:1050–1055. doi: 10.1038/nm.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmont K, McKelvey KJ, Fulcher G, Reid I, March L, Xue M, Cooper A, Jackson CJ. Treatment of chronic diabetic lower leg ulcers with activated protein C: a randomised placebo-controlled, double-blind pilot clinical trial. Int Wound J. 2015;12:422–427. doi: 10.1111/iwj.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijewardena A, Lajevardi SS, Vandervord E, Vandervord J, Lang TC, Fulcher G, Jackson CJ. Activated protein C to heal pressure ulcers. Int Wound J. 2016;13:986–991. doi: 10.1111/iwj.12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijewardena A, Vandervord E, Lajevardi SS, Vandervord J, Jackson CJ. Combination of activated protein C and topical negative pressure rapidly regenerates granulation tissue over exposed bone to heal recalcitrant orthopedic wounds. Int J Low Extrem Wounds. 2011;10:146–151. doi: 10.1177/1534734611417342. [DOI] [PubMed] [Google Scholar]
- Williams PD, Zlokovic BV, Griffin JH, Pryor KE, Davis TP. Preclinical safety and pharmacokinetic profile of 3K3A-APC, a novel, modified activated protein C for ischemic stroke. Curr Pharm Des. 2012;18:4215–4222. doi: 10.2174/138161212802430413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler EA, Sengillo JD, Sagare AP, Zhao Z, Ma Q, Zuniga E, Wang Y, Zhong Z, Sullivan JS, Griffin JH, Cleveland DW, Zlokovic BV. Blood-spinal cord barrier disruption contributes to early motor-neuron degeneration in ALS-model mice. Proc Natl Acad Sci U S A. 2014;111:E1035–1042. doi: 10.1073/pnas.1401595111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian B, Huang B. The immune response of stem cells in subretinal transplantation. Stem Cell Res Ther. 2015;6:161. doi: 10.1186/s13287-015-0167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z, Ilieva H, Hallagan L, Bell R, Singh I, Paquette N, Thiyagarajan M, Deane R, Fernandez JA, Lane S, Zlokovic AB, Liu T, Griffin JH, Chow N, Castellino FJ, Stojanovic K, Cleveland DW, Zlokovic BV. Activated protein C therapy slows ALS-like disease in mice by transcriptionally inhibiting SOD1 in motor neurons and microglia cells. J Clin Invest. 2009;119:3437–3449. doi: 10.1172/JCI38476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV, Griffin JH. Cytoprotective protein C pathways and implications for stroke and neurological disorders. Trends Neurosci. 2011;34:198–209. doi: 10.1016/j.tins.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV, Zhang C, Liu D, Fernandez J, Griffin JH, Chopp M. Functional recovery after embolic stroke in rodents by activated protein C. Ann Neurol. 2005;58:474–477. doi: 10.1002/ana.20602. [DOI] [PubMed] [Google Scholar]