Abstract
Background
Intimal hyperplasia (IH) has been historically associated historically with improper venous remodeling and stenosis after creation of an arteriovenous fistula (AVF). Recently, however, we showed that IH by itself does not explain the failure of maturation of two-stage AVFs. Herein, we seek to evaluate whether IH plays a role in the development of focal stenosisn of an AVF.
Methods
This study compares IH lesions in stenotic and nearby non-stenotic segments collected from the same AVF. Focal areas of stenosis were detected in the operating room in patients (n=14) undergoing the second-stage vein transposition procedure. The entire vein was inspected, and areas of stenosis were visually located with the aid of manual palpation and hemodynamic changes in the vein peripheral and central to the narrowing. Stenotic and non-stenotic segments were documented by photography before tissue collection (14 tissue pairs). Intimal area and thickness, intima-media thickness (IMT), and intima to media area ratio (I/M) were measured in hematoxylin and eosin stained cross-sections followed by pairwise statistical comparisons.
Results
The intimal area in stenotic and non-stenotic segments ranged from 1.25 to 11.61 mm2 and 1.29 to 5.81 mm2, respectively. There was no significant difference between these two groups (p=0.26). Maximal intimal thickness (p=0.22), maximal IMT (p=0.13), and I/M (p=0.73) were also similar between both types of segments.
Conclusions
This preliminary study indicates that postoperative IH by itself is not associated with the development of focal venous stenosis in two-stage fistulas.
Keywords: focal stenosis, arteriovenous fistula, intimal hyperplasia, maturation
TOC image
We report a similar degree of intimal hyperplasia (IH) in stenotic and nearby non-stenotic segments collected from the same AVF. The significance of this finding is that it demonstrates that postoperative IH by itself is not associated with stenosis.
INTRODUCTION
The arteriovenous fistula (AVF) is the preferred access for hemodialysis, because it has better outcomes and lesser incidence of complications than arteriovenous grafts and dialysis catheters.1,2 Use of arteriovenous fistulas has increased considerably in the United States over the last two decades,3 but, the frequency of primary failure remains dramatically elevated.4,5
Intimal hyperplasia (IH) is one of the most frequently observed vascular pathologies in patients with failed AVFs,6–9 but the notion that IH is the main cause of venous stenosis and AVF failure has been challenged by recent retrospective and prospective studies. Allon et al. demonstrated that pre-existing IH does not increase the risk of the development of stenosis after creation of a venous access creation.10 In addition, a growing body of evidence indicates that neither pre-existing nor postoperative IH by itself predisposes to primary failure of an AVF.6,11
To further understand the lack of association between the degree of IH and AVF maturation failure, in this study we evaluated whether postoperative IH plays any role in the development of focal stenosis.
MATERIALS AND METHODS
Study design
This study included 14 patients with end-stage renal disease (ESRD) over 21 years of age with a planned, two-stage AVF creation at Jackson Memorial Hospital or University of Miami Hospital. The aim was to compare the degree of IH in stenotic and non-stenotic segments obtained from the same AVF. The study design consisted in collecting a biopsy of the native vein during ther first-stage operation and discarded juxta-anastomotic AVF tissues at the time of transposition, including stenotic and nearby non-stenotic segments. A single surgeon (M.T.) performed all operative procedures, using preoperative vascular mapping of the upper extremities to plan the AVF.6 We followed the order of AVF preference recommended by the National Kidney Foundation/Kidney Disease Outcomes Quality Initiative.12 Veins that were not sclerotic visually and had a diameter ≥ 3.5 mm as determined by intraoperative measurement using a coronary dilator were used for AVF creation. Patients were followed for 3 months after transposition to assess primary failure. All sections of the study were performed according to the ethical principles of the Declaration of Helsinki and regulatory requirements at both institutions. The ethics committee and Institutional Review Board at the University of Miami approved the study.
Intraoperative assessment of AVF stenoses
At the time of AVF transposition (second-stage operation), the surgeon inspected the entire AVF for areas of stenosis with the aid of manual palpation and hemodynamic changes in the vein peripheral and central to the narrowing. Briefly, the AVF was dissected completely from the anastomosis to the upper arm. Proximal clamping of the AVF (central to the anastomosis) with the fingers resulted in engorgement of the AVF and helped differentiate a spasm from a real stenosis. On visual inspection using 3.5x magnification loupes, focal stenoses appeared typically as hourglass deformities. The AVF was inspected further for a pulse and a thrill. A clinically relevant stenosis was confirmed by the presence of a pulse peripheral to the narrowing followed by a thrill central to the narrowing. Tactile inspection also helped identify a focal stenosis by the presence of sclerosis or thickening in the area compared to the rest of the AVF. The above findings were confirmed using a coronary dilator to estimate the luminal diameter during sample collection. The luminal diameter of a focal stenosis was 3.5–4 mm compared to 6–9 mm in the rest of the AVF. All stenotic segments were located in the juxta-anastomotic region of the AVF, i.e., in the first 2 cm downstream from the arterial anastomosis.
Definitions
Macroscopic areas of stenosis were defined as the presence of vessel narrowing on intraoperative visual inspection and palpation compared to the normal AVF segment located adjacent to the stenosis (Figure 1).
Figure 1.
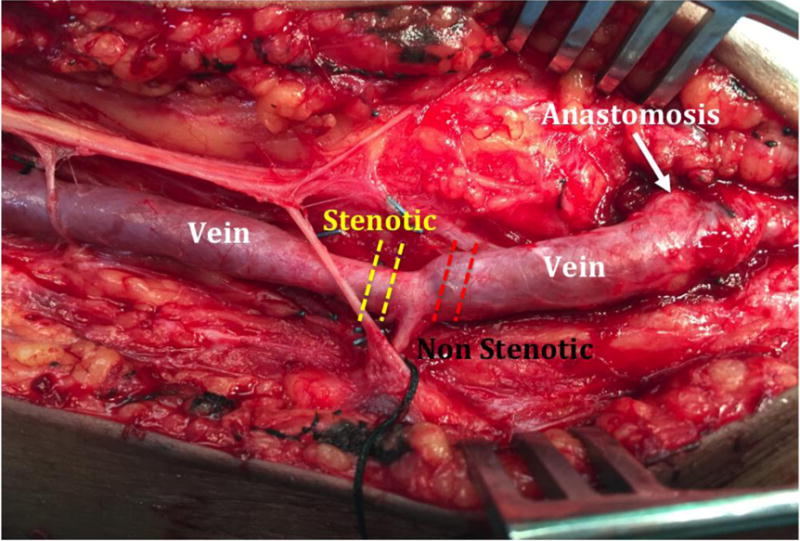
Photograph of a first-stage brachiobasilic arteriovenous fistula demarcating the locations of the focal stenotic (yellow dashed lines) and non-stenotic segments (red dashed lines) in the outflow segment.
Specimen collection and processing
Stenotic and non-stenotic segments were documented by photography before tissue collection (14 tissue pairs). The surgeon cut through the focal stenosis and removed a 2–3 mm ring where the narrowing looked the most severe. Another 2–3 mm ring was collected in the non-stenotic area, not more than 3–5 mm away from the focal narrowing. Tissue biopsies were submerged in neutral formalin immediately after collection, and de-identified and labeled with a numerical code once in the research lab. Tissues were processed, paraffin-embedded, and sectioned for histology.
Morphometric analysis
Tissue sections were stained with hematoxylin and eosin for gross histopathologic analysis. Full digital images were acquired with a Visiontek digital microscope (Sakura, Torrance, CA). Morphometric measurements were determined using ImageJ (National Institutes of Health, Bethesda, MD) by two independent observers (J.C.D, L.M.) blinded to the patient’s clinical characteristics, AVF outcomes, and classification of the tissue sections. These included intimal area and thickness, intima-media thickness (IMT), and intima to media area ratio (I/M). Intimal thickness and IMT were determined as the linear distance from the endothelium to the internal and external elastic laminae, respectively.13
Statistical analyses
Pairwise statistical analyses were performed using XLSTAT (New York, NY). Morphometric measurements were compared using Wilcoxon signed rank tests and expressed as median and interquartile range (IQR). Results were considered significant when p<0.05.
RESULTS
Demographic, clinical, and AVF characteristics of the study cohort
A total of 14 ESRD patients were included in the study. Ages ranged from 31 to 77 years (53 ± 12, mean ± SD); 10en patients were African American (71%), and nine were females (64%). All 14 patients had a diagnosis of hypertension, 10 were diabetic (71%), four presented with coronary artery disease (29%), and one was diagnosed with congestive heart failure (7%). Brachiobasilic AVFs were created in 12 patients (86%) and brachial-brachial AVFs in the remaining two (14%). The median time interval between first-stage and second-stage operations was 92 days (IQR 70–126). In agreement with previous observations,14 10 AVFs matured successfully (71%) despite the presence of a focal juxta-anastomotic stenosis in all of them.
Comparison of IH between stenotic and non-stenotic segments
The intimal area in stenotic and non-stenotic segments ranged from 1.25 to 11.61 mm2 and 1.29 to 5.81 mm2, respectively. There was no significant difference between these two groups (p=0.26; Table 1). Stenotic cross-sections had maximal intima thickness and IMT in the ranges of 0.36–2.29 mm and 0.53–2.84 mm, respectively; whereas the maximal intima thickness and IMT values for non-stenotic sections were 0.41–1.29 mm and 0.58–1.80 mm, respectively. There were no differences in any of these IH parameters between the two groups (Table 1).
Table 1.
Morphometric comparison between surgically detected stenotic and non-stenotic arteriovenous fistula segments
| Stenotic | Non-Stenotic | P-value | |
|---|---|---|---|
| Intimal Area (mm2) | 3.33 (2.29–5.16) | 3.33 (1.94–4.86) | 0.26 |
| Max. Intima Thickness (mm) | 0.98 (0.78–1.20) | 0.75 (0.54–1.08) | 0.22 |
| Min. Intima Thickness (mm) | 0.11 (0.05–0.43) | 0.09 (0.05–0.31) | 0.18 |
| Max. Intima-Media Thickness (mm) | 1.38 (1.30–1.57) | 1.14 (0.84–1.38) | 0.13 |
| Min. Intima-Media Thickness (mm) | 0.30 (0.23–0.88) | 0.37 (0.17–0.70) | 0.22 |
| Intima/Media Area Ratio | 1.00 (0.70–1.20) | 0.97 (0.63–1.18) | 0.73 |
Values are reported as median (interquartile range).
Figure 2 demonstrates the degree of similarity in IH between stenotic and non-stenotic biopsies collected from two patients. In both individuals, the native veins presented a low-moderate degree of IH, whereas AVF segments had a moderate-severe severity of IH by histology. Interestingly, these two AVFs matured successfully despite the presence of a juxta-anastomotic focal stenosis. A pairwise comparison of I/M ratios between stenotic and non-stenotic segments from the same AVF is shown in Figure 3. Nine of the 14 AVFs had similar IH in both segments (including three cases of faioure of maturation), and the I/M ratio was even les in the stenotic cross-section of five AVFs compared to the non-stenotic segments.
Figure 2.
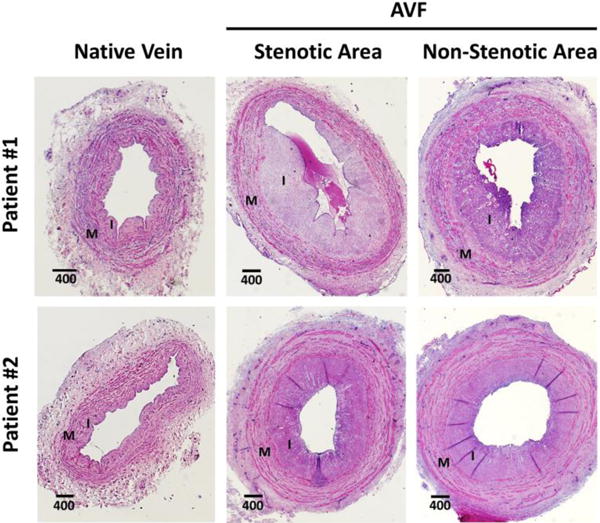
Representative hematoxylin and eosin-stained cross-sections of native veins (left panel) before anastomosis and segments of the resulting arteriovenous fistulas (AVF; right panel) at the time of transposition. Native veins present a low-moderate degree of pre-existing intimal hyperplasia (IH), whereas AVFs show moderate-severe postoperative IH. Focal stenotic and nearby non-stenotic segments from the same AVF present a similar degree of IH. I: intima, M: media. Distances are in μm.
Figure 3.
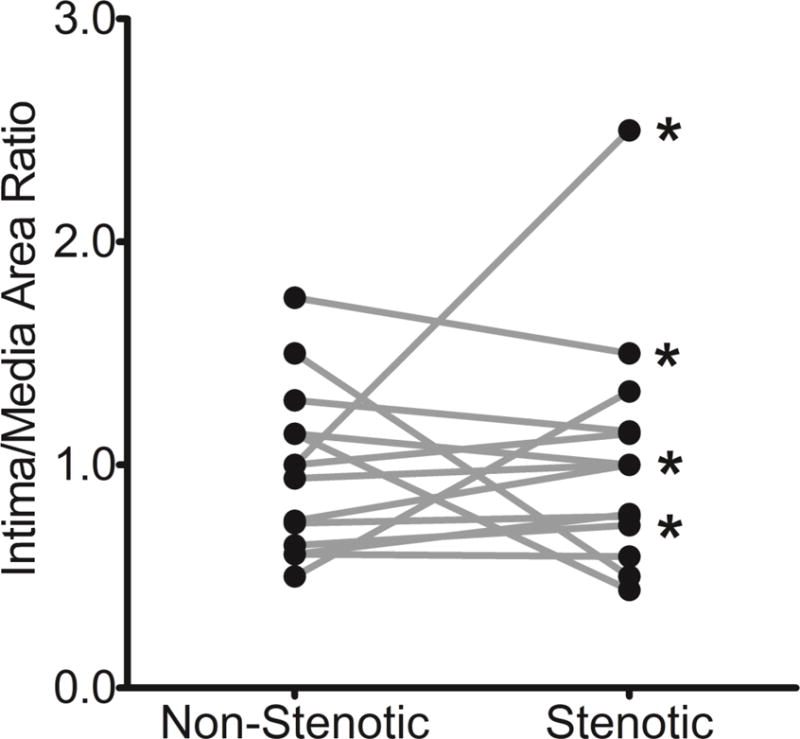
Pairwise comparison of intimal hyperplasia, expressed as intima to media area ratio, in focal stenotic and nearby non-stenotic segments from the same arteriovenous fistula (AVF; p=0.73). The asterisks indicate AVFs with maturation failure.
DISCUSSION
Despite clear evidence demonstrating the benefits of AVFs over other types of hemodialysis accesses,1,2 AVF remodeling remains an unstudied area in vascular biology. Current guidelines recommend that an AVF is created at least 6 months before the anticipated start of hemodialysis to allow sufficient time for its maturation.12 Despite this waiting period, we are still unable to prevent primary failure, partly due to an incomplete understanding of the mechanisms behind failure of maturation. This preliminary study suggests that 1) IH by itself is not responsible for the development of postoperative focal stenosis, and 2) AVF maturation can occur successfully despite the presence of focal stenosis in the juxta-anastomotic region.
Ranging from moderate to severe, all cross-sections from both stenotic and non-stenotic segments collected in our study present a similar degree of IH. This observation suggests that IH does not interfere with the outward remodeling of the vessel ; moreoever, this finding clarifies a common misconception in the vascular field that the terms stenosis and IH can be used interchangeably. Focal areas of stenosis are anatomic narrowings less than 2 cm in length and frequently observed in failed AVFs.15,16 The most typical location for these lesions is in the juxta-anastomotic region, but multiple angiographic and Doppler imaging reports have also described focal narrowings in distal segments of the outflow vein.14,16–18 Importantly, even though stenotic lesions are reported commonly in dysfunctional AVFs, they have also been observed in over 60% of well-functioning fistulas.14 The clinical outcomes in our patient cohort concur with these observations, because10 of the 14 AVFs matured successfully despite the presence of a focal stenosis in the juxta-anastomotic region.
The severity of a stenosis is reported as percent decrease in luminal area. Stenotic lesions compromising more than 50% of the lumen (also known as critical stenoses) are considered hemodynamically important, and patients are recommended to undergo an intervention.19,20 Nonetheless, the percentage decrease in luminal diameter is not represented accurately by histology sections, because this method underestimates the compressibility of the intima under conditions of supra-arterial blood flow.
Interestingly, de novo stenotic lesions are more frequent in the first 3 months after AVF creation, with 20% of them leading to occlusions and over 50% being hemodynamically important.20 This finding underscores the need for understanding the origin of these lesions in order to prevent early failure. Our work advances the current knowledge in this area and suggests that IH by itself does not explain the development of focal stenosis. Because both stenotic and non-stenotic areas collected in our study were located close to each other within the same AVF, genetic and comorbid factors can be also excluded as potential causes of focal stenosis. We can then hypothesize that focal stenotic lesions arise as a result of pre-existing abnormalities in the vein, local hemodynamic effects, or operativetrauma, such as kinking or damage to the adventitia. Inflammation and scarring of the peri-adventitial tissue has also been implicated in occlusive vascular remodeling, where perivascular adipose tissue appears to exert a protective or pathologic effect depending on the balance of pro- and anti-inflammatory mediators.21–23 A recent study demonstrated a negative correlation between the concentrations of perivascular adipokines and the increment in vein diameter during AVF maturation.23 This notion suggests that the pathophysiology of early AVF stenoses might differ from that of late stenotic lesions. Additional investigations are also needed to compare the characteristics of focal and progressive stenoses.
The main limitations of our study are the small number of samples (n=4), which increases the risk of type II statistical error, and the evaluation of only upper-arm AVFs. In addition, the qualitative intraoperative assessment of focal stenoses limits the generalizability of our results and comparisons with stenoses in already working fistulas, which are diagnosed typically using imaging methods. Despite these shortcomings, we present a systematic approach for the characterization of development of stenosis during the period of AVF maturation. This preliminary study lends support to the lack of association between IH and maturation failure,6 and suggests that IH by itself does not lead to the development of focal venous stenosis in two-stage AVFs.
Acknowledgments
This study was supported by the National Institutes of Health grant R01-DK098511 to L.H.S. and R.I.V.-P.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 12th Annual Academic Surgical Congress in Las Vegas, NV February 7–9, 2017
CONFLICT OF INTEREST
None declared.
References
- 1.Malas MB, Canner JK, Hicks CW, Arhuidese IJ, Zarkowsky DS, Qazi U, et al. Trends in incident hemodialysis access and mortality. JAMA Surg. 2015;150:441–8. doi: 10.1001/jamasurg.2014.3484. [DOI] [PubMed] [Google Scholar]
- 2.Hicks CW, Canner JK, Arhuidese I, Zarkowsky DS, Qazi U, Reifsnyder T, et al. Mortality benefits of different hemodialysis access types are age dependent. J Vasc Surg. 2015;61:449–56. doi: 10.1016/j.jvs.2014.07.091. [DOI] [PubMed] [Google Scholar]
- 3.Pisoni RL, Zepel L, Port FK, Robinson BM. Trends in US Vascular Access Use, Patient Preferences, and Related Practices: An Update From the US DOPPS Practice Monitor With International Comparisons. Am J Kidney Dis. 2015;65:905–15. doi: 10.1053/j.ajkd.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 4.Schinstock CA, Albright RC, Williams AW, Dillon JJ, Bergstralh EJ, Jenson BM, et al. Outcomes of arteriovenous fistula creation after the Fistula First Initiative. Clin J Am Soc Nephrol. 2011;6:1996–2002. doi: 10.2215/CJN.11251210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Jaishi AA, Lok CE, Garg AX, Zhang JC, Moist LM. Vascular access creation before hemodialysis initiation and use: a population-based cohort study. Clin J Am Soc Nephrol. 2015;10:418–27. doi: 10.2215/CJN.06220614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tabbara M, Duque JC, Martinez L, Escobar LA, Wu W, Pan Y, et al. Preexisting and Postoperative Intimal Hyperplasia and Arteriovenous Fistula Outcomes. Am J Kidney Dis. 2016;68:455–64. doi: 10.1053/j.ajkd.2016.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roy-Chaudhury P, Arend L, Zhang J, Krishnamoorthy M, Wang Y, Banerjee R, et al. Neointimal hyperplasia in early arteriovenous fistula failure. Am J Kidney Dis. 2007;50:782–90. doi: 10.1053/j.ajkd.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 8.Roy-Chaudhury P, Wang Y, Krishnamoorthy M, Zhang J, Banerjee R, Munda R, et al. Cellular phenotypes in human stenotic lesions from haemodialysis vascular access. Nephrol Dial Transplant. 2009;24:2786–91. doi: 10.1093/ndt/gfn708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duque JC, Tabbara M, Martinez L, Cardona J, Vazquez-Padron RI, Salman LH. Dialysis Arteriovenous Fistula Failure and Angioplasty: Intimal Hyperplasia and Other Causes of Access Failure. Am J Kidney Dis. 2017;69:147–51. doi: 10.1053/j.ajkd.2016.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allon M, Robbin ML, Young CJ, Deierhoi MH, Goodman J, Hanaway M, et al. Preoperative venous intimal hyperplasia, postoperative arteriovenous fistula stenosis, and clinical fistula outcomes. Clin J Am Soc Nephrol. 2013;8:1750–5. doi: 10.2215/CJN.02740313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vazquez-Padron RI, Allon M. New Insights into Dialysis Vascular Access: Impact of Preexisting Arterial and Venous Pathology on AVF and AVG Outcomes. Clin J Am Soc Nephrol. 2016;11:1495–503. doi: 10.2215/CJN.01860216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Kidney Foundation. KDOQI Clinical Practice Guideline for Hemodialysis Adequacy: 2015 Update. Am J Kidney Dis. 2015;66:884–930. doi: 10.1053/j.ajkd.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Lee T, Chauhan V, Krishnamoorthy M, Wang Y, Arend L, Mistry MJ, et al. Severe venous neointimal hyperplasia prior to dialysis access surgery. Nephrol Dial Transplant. 2011;26:2264–70. doi: 10.1093/ndt/gfq733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pietura R, Janczarek M, Zaluska W, Szymanska A, Janicka L, Skublewska-Bednarek A, et al. Colour Doppler ultrasound assessment of well-functioning mature arteriovenous fistulas for haemodialysis access. Eur J Radiol. 2005;55:113–9. doi: 10.1016/j.ejrad.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Clark TW, Hirsch DA, Jindal KJ, Veugelers PJ, LeBlanc J. Outcome and prognostic factors of restenosis after percutaneous treatment of native hemodialysis fistulas. J Vasc Interv Radiol. 2002;13:51–9. doi: 10.1016/s1051-0443(07)60009-8. [DOI] [PubMed] [Google Scholar]
- 16.Shin SW, Do YS, Choo SW, Lieu WC, Choo IW. Salvage of immature arteriovenous fistulas with percutaneous transluminal angioplasty. Cardiovasc Intervent Radiol. 2005;28:434–8. doi: 10.1007/s00270-003-0211-x. [DOI] [PubMed] [Google Scholar]
- 17.Beathard GA, Arnold P, Jackson J, Litchfield T, Physician Operators Forum of RMSL Aggressive treatment of early fistula failure. Kidney Int. 2003;64:1487–94. doi: 10.1046/j.1523-1755.2003.00210.x. [DOI] [PubMed] [Google Scholar]
- 18.Tonelli M, Jindal K, Hirsch D, Taylor S, Kane C, Henbrey S. Screening for subclinical stenosis in native vessel arteriovenous fistulae. J Am Soc Nephrol. 2001;12:1729–33. doi: 10.1681/ASN.V1281729. [DOI] [PubMed] [Google Scholar]
- 19.Navuluri R, Regalado S. The KDOQI 2006 Vascular Access Update and Fistula First Program Synopsis. Semin Intervent Radiol. 2009;26:122–4. doi: 10.1055/s-0029-1222455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grogan J, Castilla M, Lozanski L, Griffin A, Loth F, Bassiouny H. Frequency of critical stenosis in primary arteriovenous fistulae before hemodialysis access: should duplex ultrasound surveillance be the standard of care? J Vasc Surg. 2005;41:1000–6. doi: 10.1016/j.jvs.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 21.Takaoka M, Nagata D, Kihara S, Shimomura I, Kimura Y, Tabata Y, et al. Periadventitial adipose tissue plays a critical role in vascular remodeling. Circ Res. 2009;105:906–11. doi: 10.1161/CIRCRESAHA.109.199653. [DOI] [PubMed] [Google Scholar]
- 22.Tian Z, Miyata K, Tazume H, Sakaguchi H, Kadomatsu T, Horio E, et al. Perivascular adipose tissue-secreted angiopoietin-like protein 2 (Angptl2) accelerates neointimal hyperplasia after endovascular injury. J Mol Cell Cardiol. 2013;57:1–12. doi: 10.1016/j.yjmcc.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Mauro CR, Ding K, Xue H, Tao M, Longchamp A, Belkin M, et al. Adipose phenotype predicts early human autogenous arteriovenous hemodialysis remodeling. J Vasc Surg. 2016;63:171–6 e1. doi: 10.1016/j.jvs.2014.06.110. [DOI] [PMC free article] [PubMed] [Google Scholar]


