Abstract
Primary biliary cholangitis (PBC) is a chronic cholestatic liver disease associated with autoimmune phenomena targeting intrahepatic bile duct cells (cholangiocytes). Although PBC etiopathogenesis still remains obscure, development of anti-mitochondrial auto-antibodies against pyruvate dehydrogenase complex-E2 (PDC-E2) is a common feature. MicroRNA (miR) dysregulation occurs in liver and immune cells of PBC patients, but their functional relevance is largely unknown. We previously reported that miR-506 is overexpressed in PBC cholangiocytes and directly targets both Cl−/HCO3− anion exchanger 2 (AE2) and type III inositol 1,4,5-trisphosphate receptor (InsP3R3), leading to cholestasis. Here, the regulation of miR-506 gene expression and its role in cholangiocyte pathophysiology and immune activation was studied. Several pro-inflammatory cytokines overexpressed in PBC livers [such as IL8, IL12, IL17, IL18 and TNFα] stimulated miR-506 promoter activity in human cholangiocytes, as revealed by luciferase reporter assays. Experimental overexpression of miR-506 in cholangiocytes dysregulated the cell proteomic profile (by mass spectrometry), affecting proteins involved in different biological processes including mitochondrial metabolism. In cholangiocytes, miR-506: i) induced dedifferentiation with downregulation of biliary and epithelial markers together with upregulation of mesenchymal, pro-inflammatory and pro-fibrotic markers; ii) impaired cell proliferation and adhesion; iii) increased oxidative and endoplasmic reticulum (ER) stress; iv) caused DNA damage; and v) sensitized to caspase-3-dependent apoptosis induced by cytotoxic bile acids. These events were also associated with impaired energy metabolism in mitochondria (proton leak and less ATP production) and PDC-E2 overexpression. Co-culture of miR-506 overexpressing cholangiocytes with PBC immunocytes induced activation and proliferation of PBC immunocytes. Conclusion: different pro-inflammatory cytokines enhance the expression of miR-506 in biliary epithelial cells. MiR-506 induces PBC-like features in cholangiocytes and promotes immune activation, representing a potential therapeutic target for PBC patients.
Keywords: cholangiocytes, microRNAs, anion exchanger 2 (AE2), pyruvate dehydrogenase complex E2 (PDC-E2), pathogenesis
Introduction
Primary biliary cholangitis (PBC) is a chronic cholestatic liver disease associated with autoimmune phenomena targeting small and medium intrahepatic bile ducts.(1, 2) It is characterized by progressive impairment and destruction of bile duct epithelial cells (i.e. cholangiocytes) together with increased portal inflammation and fibrosis. In the absence of treatment, it may progress to liver cirrhosis needing transplantation.(1, 2) PBC is considered a multifactorial disease but the etiologies still remain obscure. It mainly affects middle-aged women and most of the patients (~90%) develop anti-mitochondrial auto-antibodies (AMA) specific against the E2 component of the pyruvate dehydrogenase complex (PDC-E2).(1, 2) Despite these autoimmune events, treatment of PBC patients with classical immunosuppressants is inefficient and the first line treatment approved by the food and drug administration (FDA) is the daily administration of ursodeoxycholic acid (UDCA), which improves prognosis in ~2/3 of patients when treated in early stages of the disease.(1, 2) UDCA is a hepatoprotective bile acid that promotes choleresis by stimulating the hepatobiliary secretion of bicarbonate, which further induces the alkalinization and fluidization of bile and prevents the damaging effect of hydrophobic bile acids on the biliary epithelium.(1, 2)
Cholestasis in PBC patients is linked to impaired biliary bicarbonate secretion.(3, 4) The main bicarbonate extruder in normal human cholangiocytes is the Cl−/HCO3− anion exchanger 2 (AE2/SLC4A2), which is located in the apical membrane promoting secretin-stimulated biliary bicarbonate secretion and regulating the intracellular pH (pHi) homeostasis.(5–7) PBC patients show a lack of response to secretin associated with decreased expression of AE2 in cholangiocytes, which results in cholestasis.(8) However, UDCA treatment restores the secretin response and improves cholestasis in PBC patients. The etiopathogenic role of the characteristic AE2 downregulation in both liver and peripheral blood mononuclear cells (PBMCs) of PBC patients is highlighted by the fact that Ae2−/− mice spontaneously develop several hepatobiliary and immunological PBC-like features, including AMA specific against PDC-E2.(9) The expression of AE2 in cholangiocytes becomes upregulated under the combination of UDCA and glucocorticoids, treatment employed for patients that do not respond to UDCA monotherapy.(6) On the other hand, type III inositol 1,4,5-trisphosphate receptor (InsP3R3) is an integral membrane protein located in the subapical portion of the endoplasmic reticulum (ER) of cholangiocytes that functions as a major intracellular calcium (Ca2+i) release channel and its activation promotes biliary bicarbonate secretion.(10, 11) Like AE2, the expression of InsP3R3 was also found downregulated in PBC cholangiocytes promoting cholestasis. Notably, the characteristic downregulation of both AE2 and InsP3R3 in PBC cholangiocytes is mediated, at least partially, by microRNA-506 (miR-506), which directly targets both AE2 and InsP3R3 mRNAs in cholangiocytes leading to impaired biliary secretory functions.(8, 12) Therefore, miR-506 seems to have a pivotal role in the etiopathogenesis of PBC but the mechanisms regulating its expression in cholangiocytes and the direct functional effects of miR-506 in cholangiocytes are still unknown.
In this study, we have investigated the role of different factors –such as pro-inflammatory cytokines, bile acids and estrogens– in the regulation of miR-506 expression in cholangiocytes. Moreover, the effect of miR-506 in cholangiocyte pathophysiology and in PBC immune regulation was studied.
Materials and methods
Luciferase reporter constructs and assays
Three fragments of different size of the human miR-506 gene (hsa-miR-506; NCBI Gene ID: 574511) promoter [i.e. 3229, 1936 or 993 bp of the 5′-flanking region of hsa-miR-506 (Z1-hsa-miR-506pr, Z2-hsa-miR-506pr and Z3-hsa-miR-506pr, respectively)] were cloned in a luciferase expression vector as described in Supplementary data (c.f. Supplementary Figures 1A, B and Supplementary Table 1). Next, non-tumor SV40-immortalized human cholangiocytes (i.e. H69, a gift from Dr. D. Jefferson, Tufts University, Boston, MA) were transfected with Z1, Z2 or Z3 recombinant vectors using Lipofectamine 2000 (Life Technologies) as described in Supplementary data. The transfection mix was replaced 6h later by DMEM+10% FBS alone or together with different pro-inflammatory cytokines [i.e. interleukins (IL) 1β, 6, 8, 12, 17, 18, tumor necrosis factor alpha (TNFα), and interferon gamma (IFNγ)], pro-fibrotic factors [i.e. transforming growth factor beta 1 (TGFβ1)], estrogens (i.e. 17β-estradiol), glucocorticoids [i.e. dexamethasone (DEX)], growth factors [i.e. epidermal growth factor (EGF)] and bile acids [i.e. cholic acid (CA), ursodeoxycholic (UDCA) and tauroursodeoxycholic (TUCA)] (Supplementary Table 2). Then, 24h after transfection, 50 µL of Lysis Buffer was added into each well and the luciferase activity was assessed (in 20 uL of cell homogenate) using the Luciferase Assay Kit E151A (Promega). Luciferase activity was measured in a NOVOstar Apparatus (BMG LABTECH) and the resultant values were normalized to the total protein concentration.
Stable overexpression of miR-506 in human cholangiocytes
H69 human cholangiocytes were stably transfected with recombinant vectors containing miR-506 (H69-miR-506) or miRNA-negative (H69-miR-neg) control sequence (Thermo Fisher Scientific), or just with vehicle (H69), as we previously described.(12) Then, transfected cells were continuously selected with blasticidin (Invitrogen) in fully supplemented DMEM/F-12 medium.(13)
Gene and miR-506 expression
Quantification of gene and miR-506 expression was performed as previously described(8) and as described in Supplementary data. Primer sequences are shown in Supplementary Table 3.
Mass spectrometry
Shotgun comparative proteomic analysis of H69, H69-miR-neg and H69-miR-506 cells was performed using iTRAQ (isobaric Tags for Relative and Absolute Quantitation).(14) Peptide labeling, peptide fractionation and mass-spectrometry analysis were performed as previously described(15) (see Supplementary data for technical details). After MS/MS analysis, protein identification and relative quantification were performed with the ProteinPilot™ software (version 4.5; Sciex) using the Paragon™ algorithm as the search engine.(16) Although relative quantification and statistical analysis were provided by the ProteinPilot software, additional 1.3-fold change cutoff for all iTRAQ ratios (ratio <0.77 or >1.3) and a p-value lower than 0.05 were selected to classify proteins as up- or down-regulated. Functional analysis of proteins was determined by gene ontology (GO) enrichment using the Panther Classification System database (http://pantherdb.org/).
Immunoblotting
The analysis of protein expression was performed as described in Supplementary data and using the antibodies listed in Supplementary Table 4.
Cell proliferation, adhesion and migration
The evaluation of cell proliferation, adhesion and migration was carried out in H69, H69-miR-neg and H69-miR-506 cholangiocytes as described in Supplementary data.
Oxidative stress
Dihydroethidium (DHE) staining was used to detect reactive oxygen species (ROS) in H69, H69-miR-neg and H69-miR-506 cholangiocytes. For this purpose, 2×104 cells per well were seeded in coverslips in 24-well plates and these were grown for 48h. Cells were stained with 15 µM DHE (Molecular Probes, ThermoFisher) for 10 min at 37°C in darkness, fixed with 1% paraformaldehyde in PBS and mounted using Vectashield (Vector Laboratories). Pictures were taken using a fluorescence microscope (Leica DM IRB).
Cell viability and apoptosis
The cytotoxicity of the hydrophobic bile acids chenodeoxycholic acid (CDCA: 200 µM, Sigma) and glycochenodeoxycholic (GCDCA: 750 µM, Sigma) was evaluated in H69, H69-miR-neg and H69-miR-506 cholangiocytes. Additionally, caspase-mediated apoptosis and necroptosis were investigated incubating cells with specific inhibitors (zVAD-fmk and necrostatin-1, respectively, both from Calbiochem, at 20 µM). Cell viability was evaluated by using both CellTiter-Glo® (Promega) and WST-1 (Roche) assays, following manufacturer´s instructions. Apoptotic rates of cholangiocytes were measured by flow cytometry using Annexin V Alexa Fluor 594 conjugate (Thermo Fisher Scientific) and Propidium Iodide (Life Technologies) in a Guava Easycyte 8HT apparatus. See Supplementary data for further details.
Mitochondrial metabolic activity
Oxygen consumption and extracellular acidification rates (OCR and ECAR, respectively) were measured in an XF96 Extracellular Flux Analyzer (Seahorse Bioscience) using the XF Cell Mito Stress Test Kit following manufacturer’s instructions.
ATP production
Levels of ATP in H69, H69-miR-neg and H69-miR-506 cholangiocytes were measured using the ATP Assay Kit (Abcam). Cells were harvested and 3×106 cholangiocytes were processed following manufacturer’s instructions. Triplicates were used in each assay. Absorbance was measured at 570 nm in a Multiskan Ascent spectrophotometer (Thermo).
Immunofluorescence
Expression of PDC-E2 was evaluated by immunofluorescence in H69, H69-miR-neg and H69-miR-506 cholangiocytes as described in Supplementary data.
Co-culture of human cholangiocytes with peripheral blood mononuclear cells (PBMCs)
H69, H69-miR-neg and H69-miR-506 human cholangiocytes were co-cultured with PBMCs isolated from both normal and PBC individuals, and then immune activation (CD25; BD Biosciences) and proliferation (CellTrace™CFSE Cell Proliferation Kit; Invitrogen) were evaluated by flow cytometry as described in Supplementary data. The supernatants of the different cell cultures and cell co-cultures were harvested and cytokine levels (i.e. IL17A and IL23) were evaluated using Milliplex Map human high sensitivity T cell panel – Immunology multiplex assay (Millipore), following manufacturer’s instructions. The research protocol was approved by the Ethical Committee for Clinical Research of the Donostia University Hospital, and the patient signed a written consent for the use of the blood sample for biomedical research.
Statistical Analysis
Results were statistically analyzed using the GraphPad Prism 6 statistical software (San Diego, USA). Data are shown as means ± standard error of the mean (SEM). For comparisons between two groups, parametric t-Student test or non-parametric Mann-Whitney test were used. For comparisons between more than two groups, parametric One-Way analysis of variance (ANOVA) or non-parametric Kruskal-Wallis tests followed by a posteriori Bonferroni or Dunns tests were used respectively.
Results
Regulation of miR-506 promoter activity in human cholangiocytes
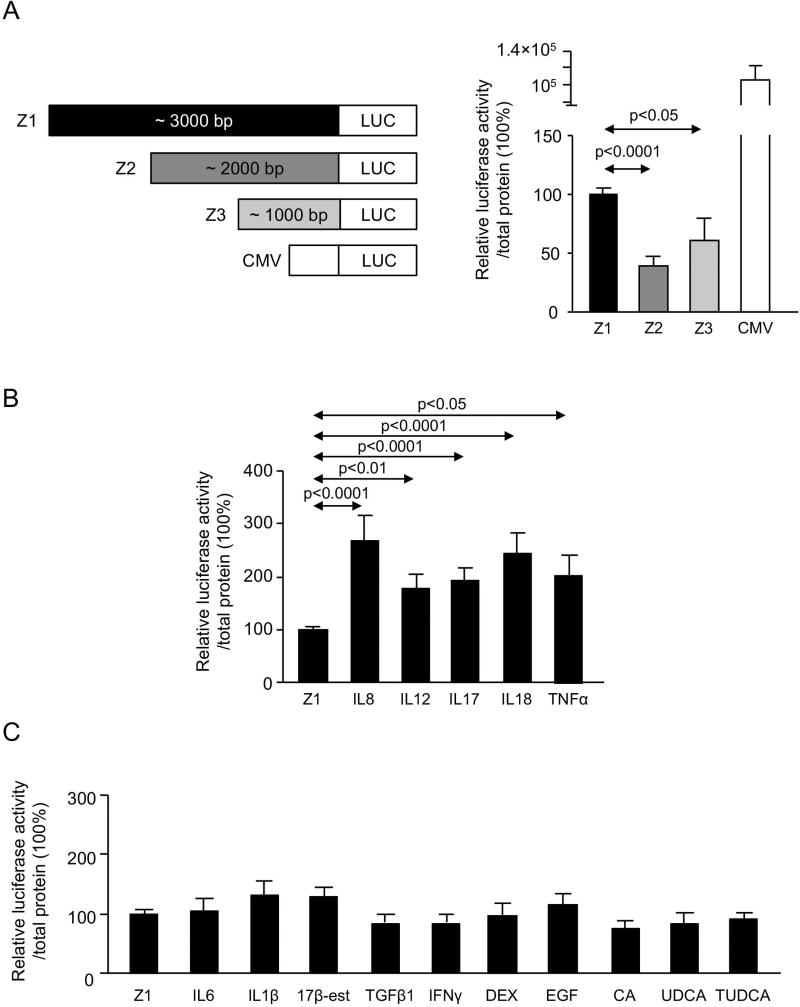
We previously reported that miR-506 expression is upregulated in PBC cholangiocytes compared to normal human cholangiocytes.(8) Here, the regulatory activity of miR-506 promoter was evaluated in human cholangiocytes using recombinant luciferase reporter vectors containing a fragment of the promoter region. Three fragments of different length of the sequence located immediately 5’-upstream of miR-506 were cloned and assayed [i.e. Z1 (3229 bp), Z2 (1936 bp) and Z3 (993 bp)] (Figure 1A and Supplementary Figure 1) in the presence or absence of different pro-inflammatory, pro-fibrotic and/or pro-mitotic molecules found overexpressed in PBC livers. As positive control, a recombinant vector containing the cytomegalovirus (CMV) promoter was used (Figure 1A). Under basal conditions, the recombinant luciferase vector containing the Z1 (the longest fragment) miR-506 promoter showed higher expression of luciferase compared to both Z2 and Z3 fragments, whereas no differences in the activities were observed between Z2 and Z3 fragments (Figure 1A). The presence of pro-inflammatory cytokines IL8, IL12, IL17, IL18 or TNFα all upregulated the luciferase expression in human cholangiocytes transfected with the Z1 recombinant vector (Figure 1B). Of note, these effects were absent in cells transfected with Z2- or Z3-promoter fragment (Supplementary Figure 3). On the other hand, other pro-inflammatory (i.e. IL6, IL1β and IFNγ) and pro-fibrotic (i.e. TFGβ1) cytokines did not affect miR-506 Z1-promoter activity in cholangiocytes (Figure 1C). Finally, the presence of bile acids (i.e. CA, UDCA and TUDCA), estrogens (i.e. 17β-estradiol), glucocorticoids (i.e. DEX) and growth factors (i.e. EGF) neither affect the Z1-promoter activity in human cholangiocytes (Figure 1C).
Figure 1. MiR-506 promoter activity in cholangiocytes.
(A) Three fragments of different length (i.e. 3229, 1936 and 993 bp) located 5’-upstream hsa-miR-506, named as Z1, Z2 and Z3 respectively, were cloned upstream of the beginning of transcription of firefly luciferase (Luc2) coding sequence. Z1 promoter show higher luminescent levels compared to Z2 and Z3 (n=10). CMV promoter was used as a positive control (n=10). (B) Interleukins 8, 12, 17 and 18, as well as TNFα, increase the luciferase activity of promoter Z1 (n=10). (C) Interleukin 6 and 1β, 17β-estradiol, TGFβ1, IFNγ, DEX, EGF and bile acids (CA, UDCA, TUDCA) did not influence Z1 promoter luciferase activity (n=10).
Effect of miR-506 on the proteomic profile of human cholangiocytes
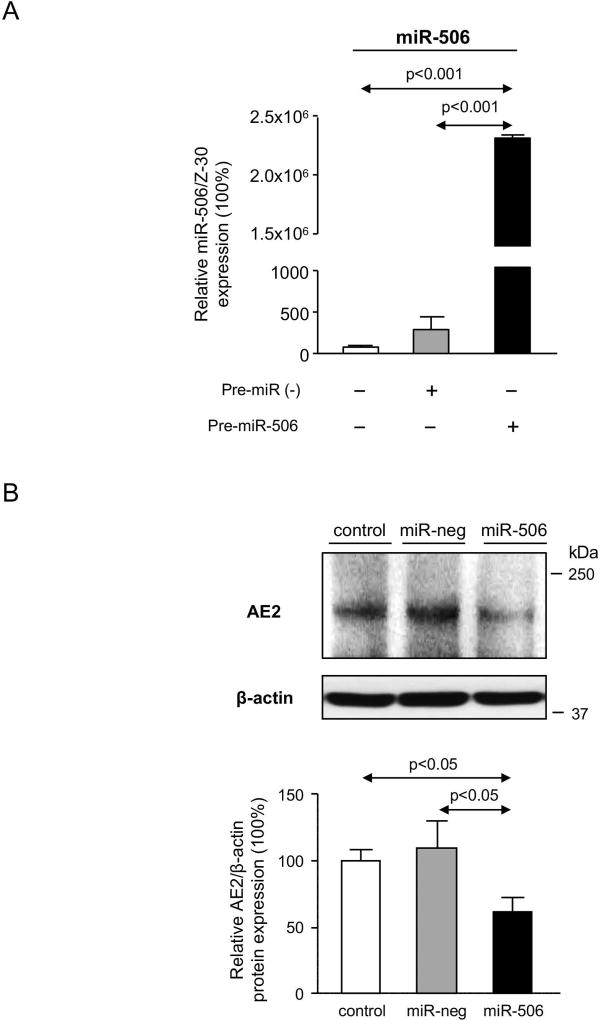
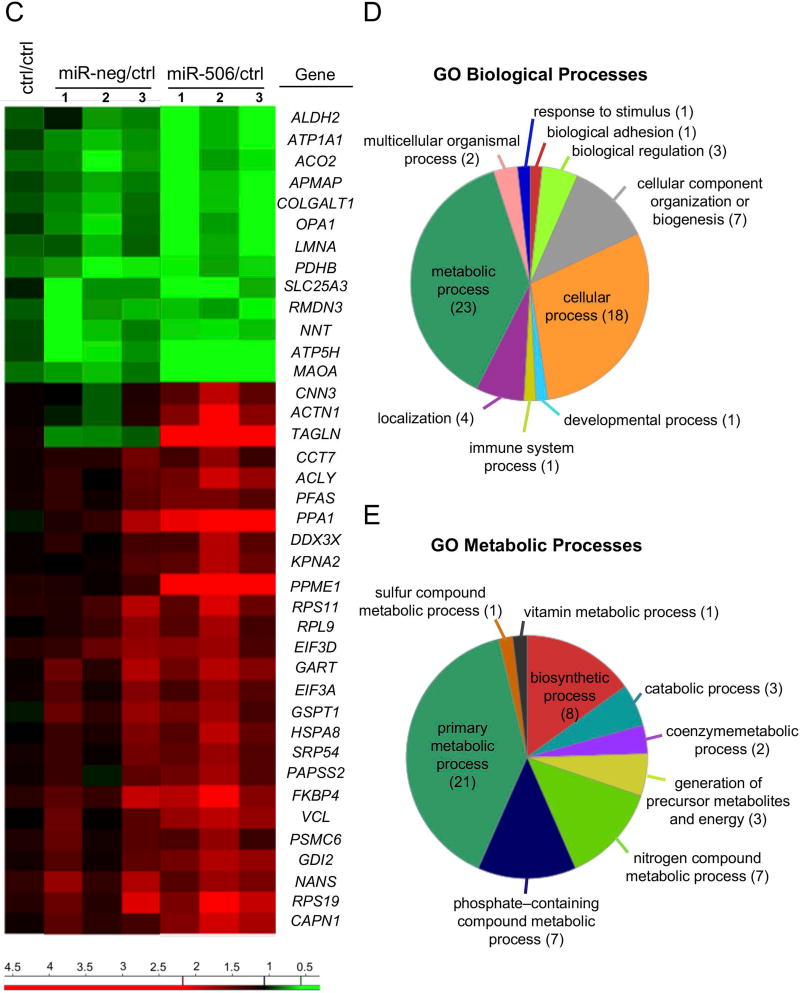
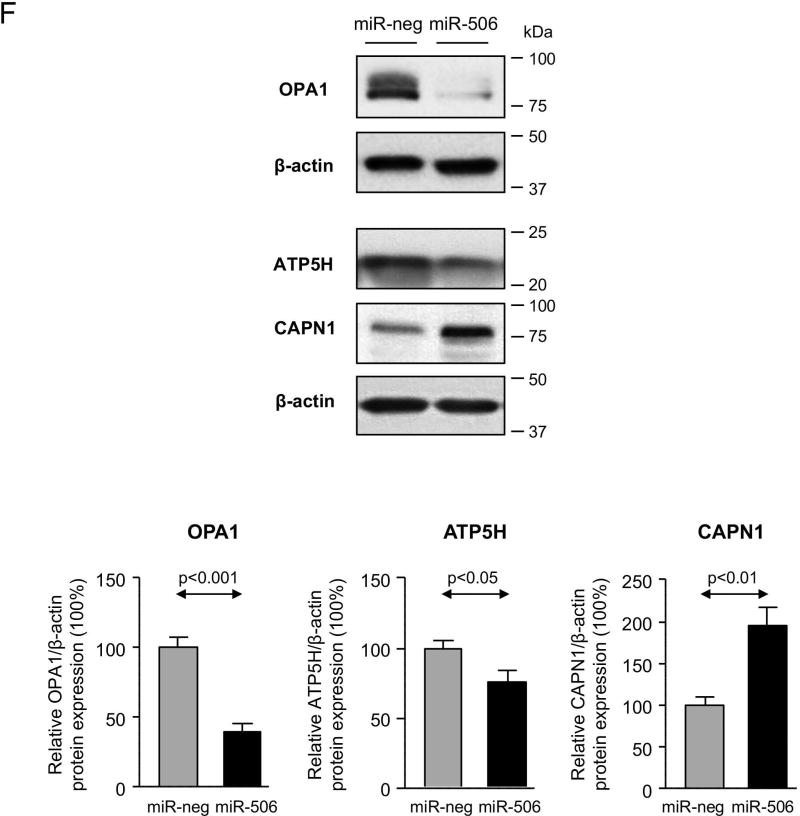
H69 cholangiocytes overexpressing miR-506 (H69-miR-506) or a negative control (H69-miR-neg) sequence under the regulation of a CMV promoter were generated. We confirmed by qPCR that the expression of the miR-506 precursor (pre-miR-506) resulted in increased expression of the mature miR-506 sequence compared to cells transfected with the pre-miR-neg or vehicle (Figure 2A). We previously reported that transient transfection of miR-506 in H69 cholangiocytes resulted in downregulation of AE2 protein expression and activity.(8) As predicted, stable transfection of H69 cholangiocytes with recombinant vectors that overexpress pre-miR-506 also diminished AE2 protein expression compared to cells transfected with the pre-miR-neg or vehicle (Figure 2B). To elucidate the pathophysiological impact of miR-506 in cholangiocytes, a proteomic analysis was performed in H69, H69-miR-neg and H69-miR-506 cells. Overexpression of miR-506 in human cholangiocytes prompted the dysregulation of multiple proteins compared to control conditions (Figure 2C). The dysregulated proteins are involved in different biological processes such as biological adhesion (i.e. COLGALT1), biological regulation (i.e. ATP1A1, DDX3X and KPNA2) and cellular component organization or biogenesis (i.e. GSPT1, RPS19, OPA1, HSPA8, LMNA, CCT7 and ACTN1), among others, but mainly in mitochondrial metabolic processes (i.e. ALDH2, ACO2, OPA1, PDHB, SLC25A3, NNT, ATP5H and ACLY) (Figures 2C–E). In particular, the expression of three central mitochondrial proteins was validated by immunoblotting confirming the downregulation of dynamin-like 120 kDa protein (OPA1) and ATP synthase subunit D (ATP5H), as well as the upregulation of calpain 1 (CAPN1) in H69-miR-506 cells compared to controls (Figure 2F).
Figure 2. Proteomic profile associated to miR-506 overexpression in cholangiocytes.
(A) H69 cells transfected with miR-506 precursor have increased mRNA expression levels of mature miR-506 compared to H69 cells transfected with a negative control vector (miR-neg) or vehicle (H69). Z-30 was used as housekeeping control. (B) Representative immunoblot showing that miR-506 overexpression in H69 cells leads to downregulation of AE2 protein expression compared to controls. Bar-graph shows AE2 quantification (n=5). (C) Heatmap of the differentially-expressed proteins and fold-change compared to H69 control cells. (D–E) Functional classification of dysregulated proteins using the Panther Classification System database (http://pantherdb.org/), and classifications based on (D) biological or (E) metabolic processes. (F) Representative immunoblots and corresponding quantification of OPA1, ATP5H and CAPN1 expression (n=4–8).
Role of miR-506 on the biliary phenotype and on cholangiocyte proliferation, adhesion and migration
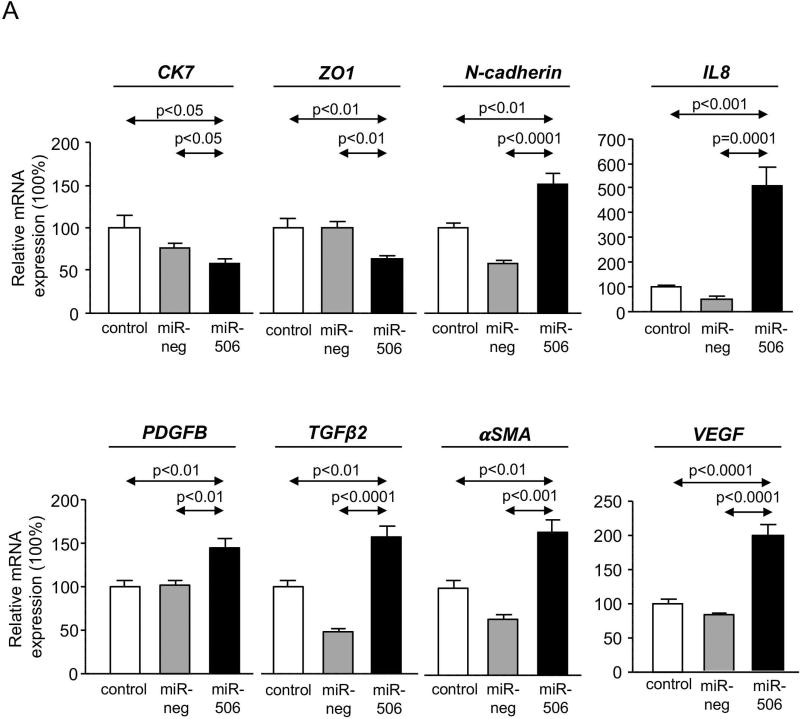
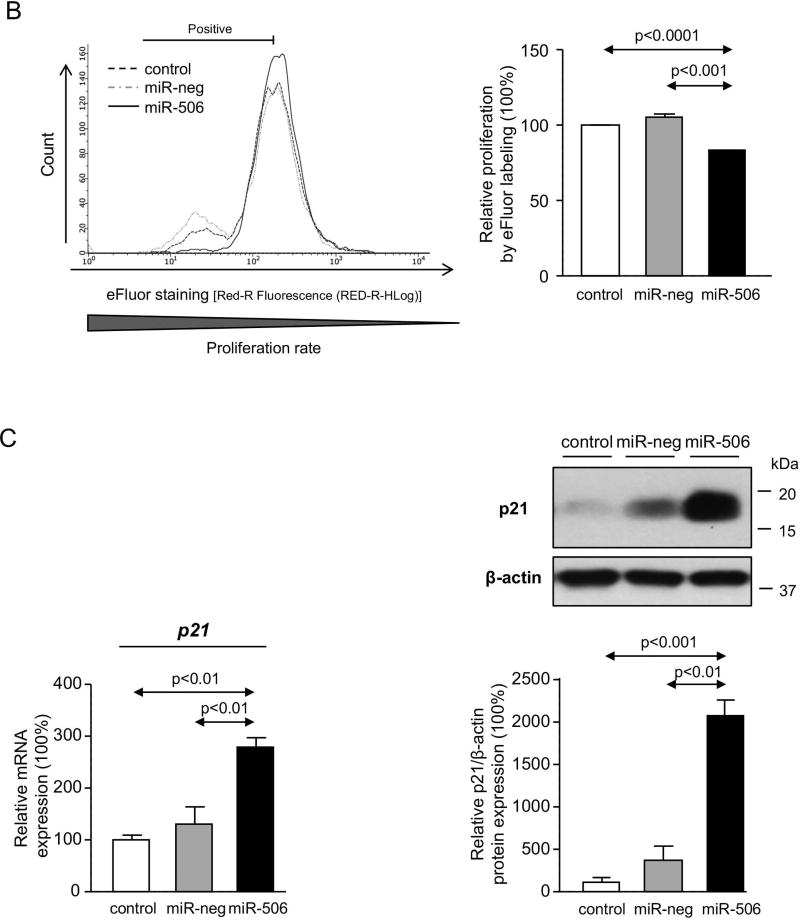
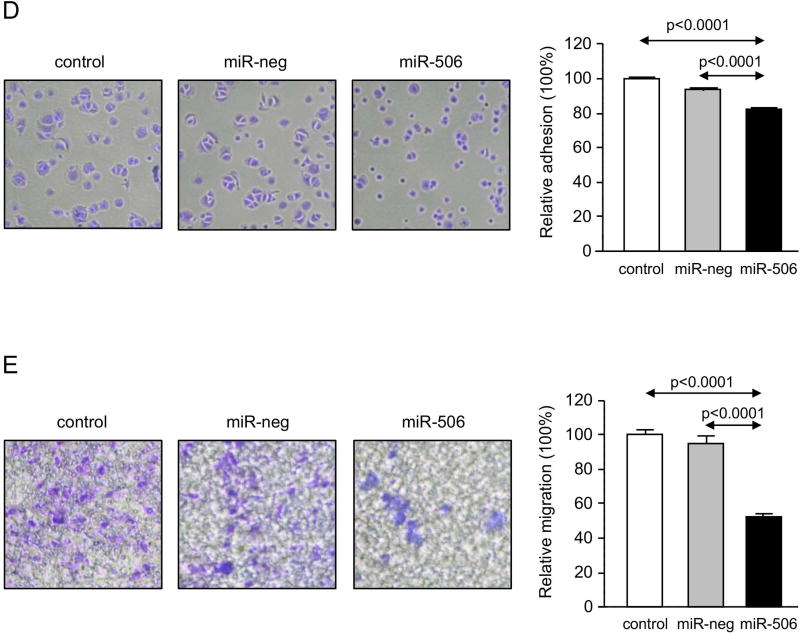
PBC cholangiocytes are characterized by dedifferentiation, linked to decreased expression of biliary(17) and epithelial markers and acquisition of pro-inflammatory, pro-fibrotic and mesenchymal(18) markers. In this regard, H69-miR-506 cells showed downregulation of biliary [i.e. cytokeratin 7 (CK7)] and epithelial [i.e. zonula occludens-1 (ZO1)] markers and increased expression of pro-inflammatory (i.e. IL8 and VEGF), pro-fibrotic (PDGFB, TGFβ2, and αSMA) and mesenchymal (i.e. N-cadherin) markers compared to controls (Figure 3A). On the other hand, miR-506 decreased cell proliferation associated with upregulation of the cell cycle inhibitor p21 at mRNA and protein level compared to controls (Figures 3B and C). This altered phenotype also resulted in decreased adhesion and migration of cholangiocytes (Figures 3D and E).
Figure 3. MiR-506 induces cholangiocyte dedifferentiation and inhibits cell proliferation, adhesion and migration.
(A) Bar-graph showing the mRNA expression levels of biliary, epithelial, mesenchymal, pro-inflammatory and pro-fibrotic markers compared to controls (n=6). (B) Representative flow cytometry-based histograms and quantification of proliferation (n=3). (C) p21 mRNA expression (n=6) (with GAPDH used as housekeeping control) and representative immunoblot of p21 protein expression. Bar-graph shows p21 quantification, using β-actin as housekeeping control (n=3). (D) Representative images and corresponding quantification of the adhesion properties of H69, H69-miR-neg and H69-miR-506 cells (n=8). (E) Representative images and corresponding quantification of cell migration analysis using transwell assays (n=5).
Involvement of miR-506 in cholangiocyte stress and apoptosis
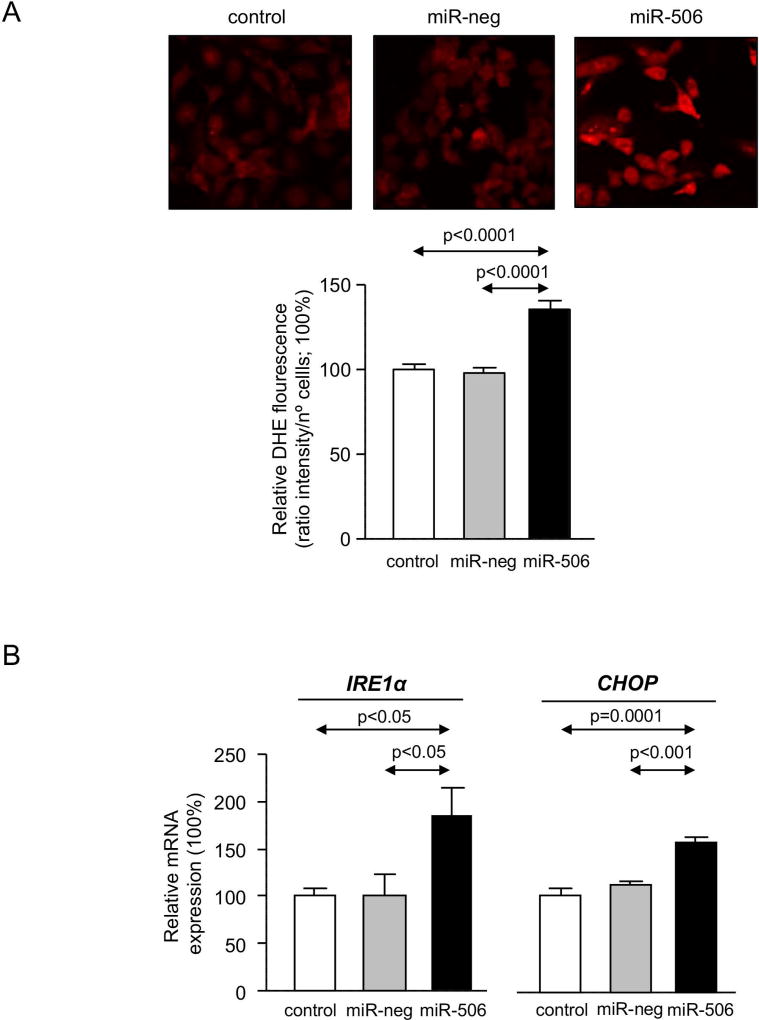
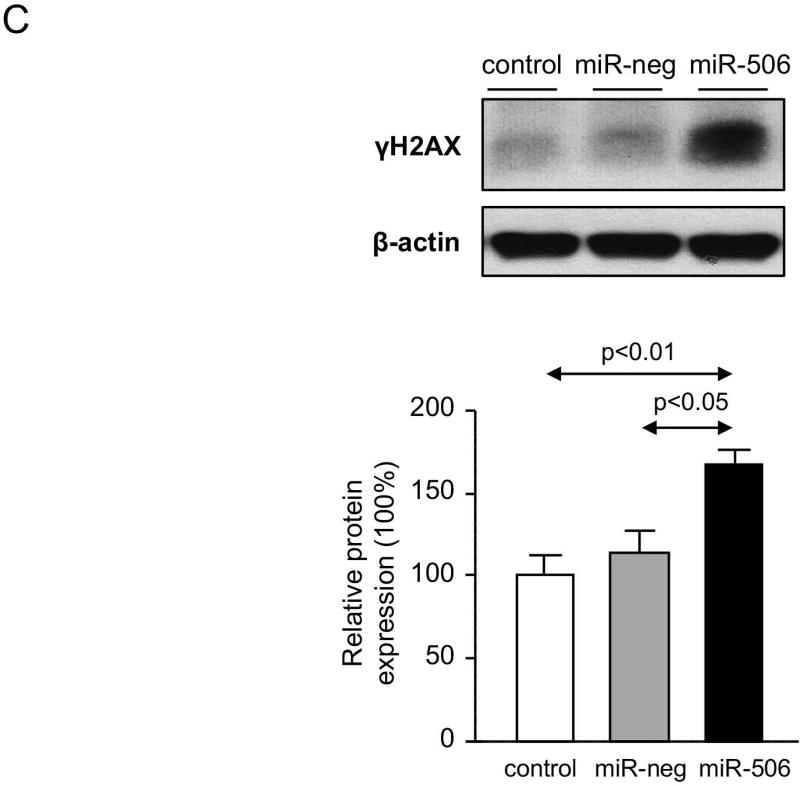
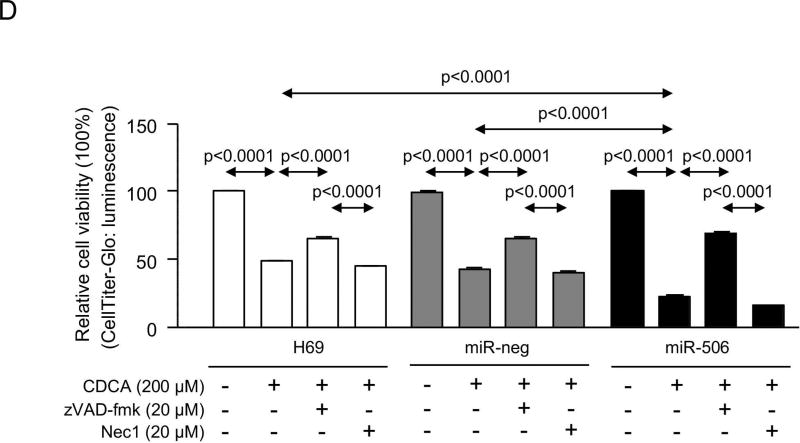
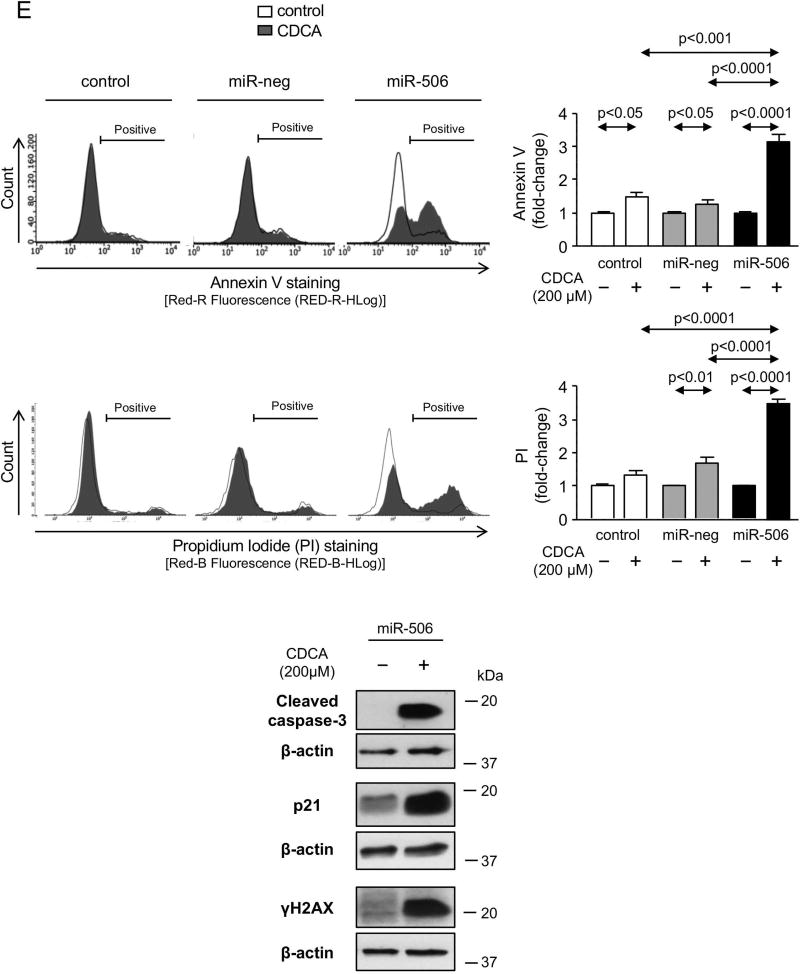
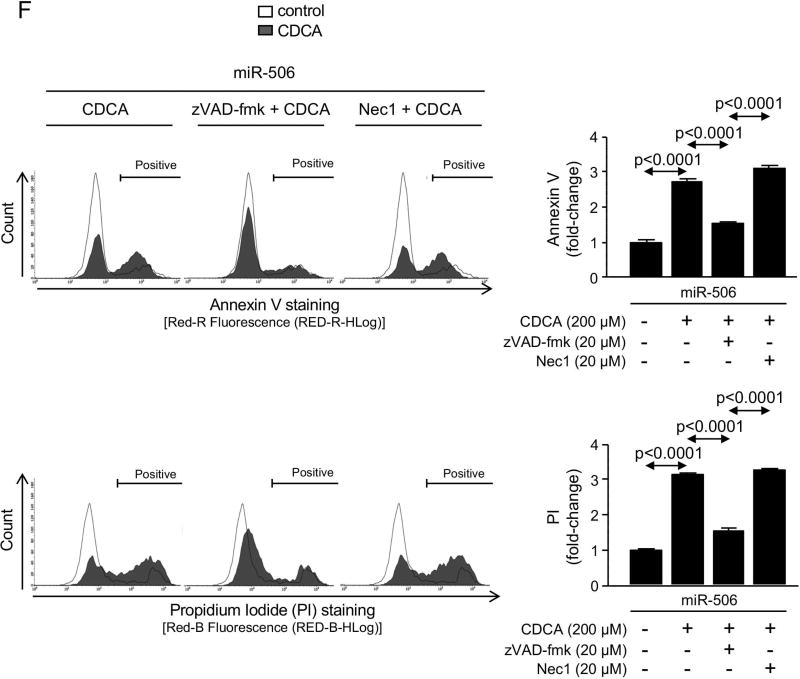
PBC cholangiocytes are characterized by cellular stress(19) and increased apoptosis.(20, 21) These PBC features are promoted by downregulation of AE2 and InsP3R3 in cholangiocytes. Thus, the lack of AE2 in PBC cholangiocytes sensitizes the cells to apoptosis induced by cytotoxic apolar hydrophobic bile acids,(22) and the downregulation of InsP3R3 leads to altered ER-related Ca2+ signaling.(10, 12, 23) Since miR-506 directly targets both AE2 and InsP3R3 mRNAs, cellular stress and apoptosis were evaluated in H69-miR-506 cells and controls. MiR-506 increased the levels of reactive oxidative species (ROS) in cholangiocytes (Figure 4A) and upregulated the expression of two key endoplasmic reticulum (ER) stress markers such as IRE1α and CHOP (Figure 4B) compared to controls; in contrast, the expression of markers of other ER stress pathways such as ATF6, PERK or XBP1 were found unchanged between the experimental groups (Supplementary Figure 4). All these pathological events were associated with protein upregulation of the DNA damage marker γH2AX (Figure 4C) but did not affect the baseline apoptosis of cholangiocytes compared to controls (Supplementary Figure 5). However, notably, miR-506 sensitized cholangiocytes to cell death induced by the toxic bile acids CDCA or GCDCA, measured by cell viability determinations [i.e. CellTiter-Glo (Figure 4D) and WST-1 (Supplementary Figure 6)] and flow-cytometry-based apoptotic assays [i.e. Annexin-V and Propidium Iodide (Figure 4E and Supplementary Figure 7)], compared to controls. This bile acid-induced cell death is mainly mediated by apoptosis, as the caspase inhibitor zVAD-fmk resulted in protection against the CDCA adverse effects in cholangiocytes and the necroptosis inhibitor necrostatin-1 had no effects (Figures 4D, F and Supplementary Figure 6). In this regard, CDCA stimulated p21 expression, caspase-3 activity and γH2AX expression in H69-miR-506 cells (Figure 4E).
Figure 4. MiR-506 induces DNA damage in cholangiocyte and sensitizes to toxic bile acid-induced apoptosis.
(A) Representative fluorescence microscopy images and quantification of DHE staining (n=57–59 cells in each group). (B) mRNA expression of ER stress markers IRE1α and CHOP (n=6). GAPDH was used as housekeeping control. (C) Representative immunoblot of γH2AX. Bar-graph showing quantification relative to β-actin (n=4). (D) Luminescence-based cholangiocyte viability assay (Cell Titer-Glo) under the presence of CDCA alone or in combination with a caspase inhibitor (zVAD-fmk) or a necroptosis inhibitor (Nec-1) (n=5). (E) Flow cytometry-based apoptosis images and quantification of Annexin V and Propidium Iodide staining under the presence of CDCA (n=6). Immunoblots of cleaved caspase-3, p21 and γH2AX in H69-miR-506 cells in the presence or absence of CDCA. β-actin was used as housekeeping control. (F) Flow cytometry-based apoptosis of H69-miR-506 cells in the presence of CDCA alone or in combination with zVAD-fmk or Nec-1 (n=4). Immunoblot of cleaved caspase-3 in H69-miR-506 cells under the presence of CDCA alone or in combination with a caspase inhibitor (zVAD-fmk). β-actin was used as housekeeping control.
Role of miR-506 in the mitochondrial energetic metabolism in cholangiocytes
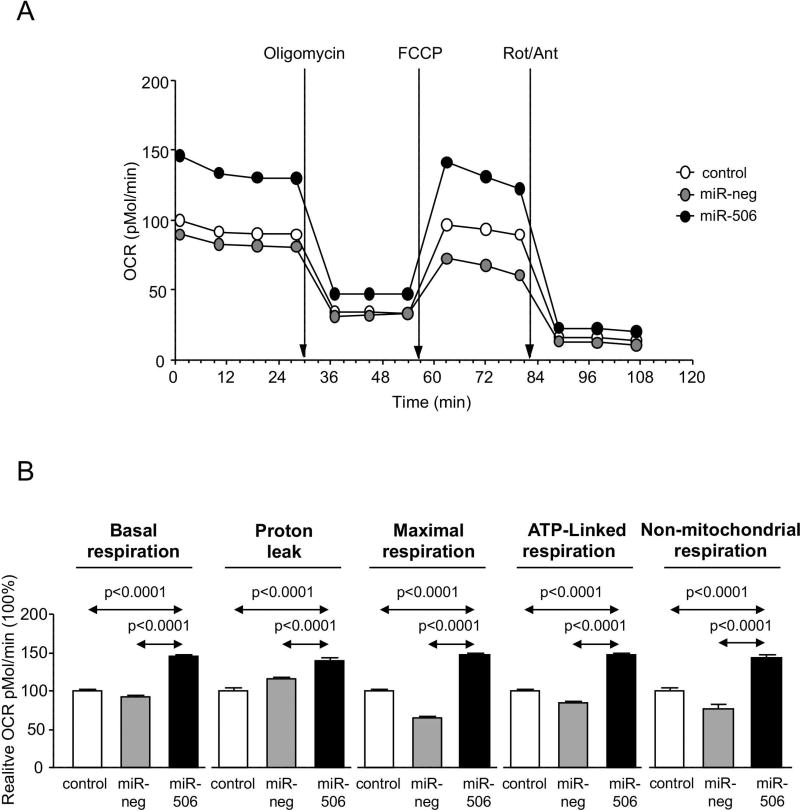
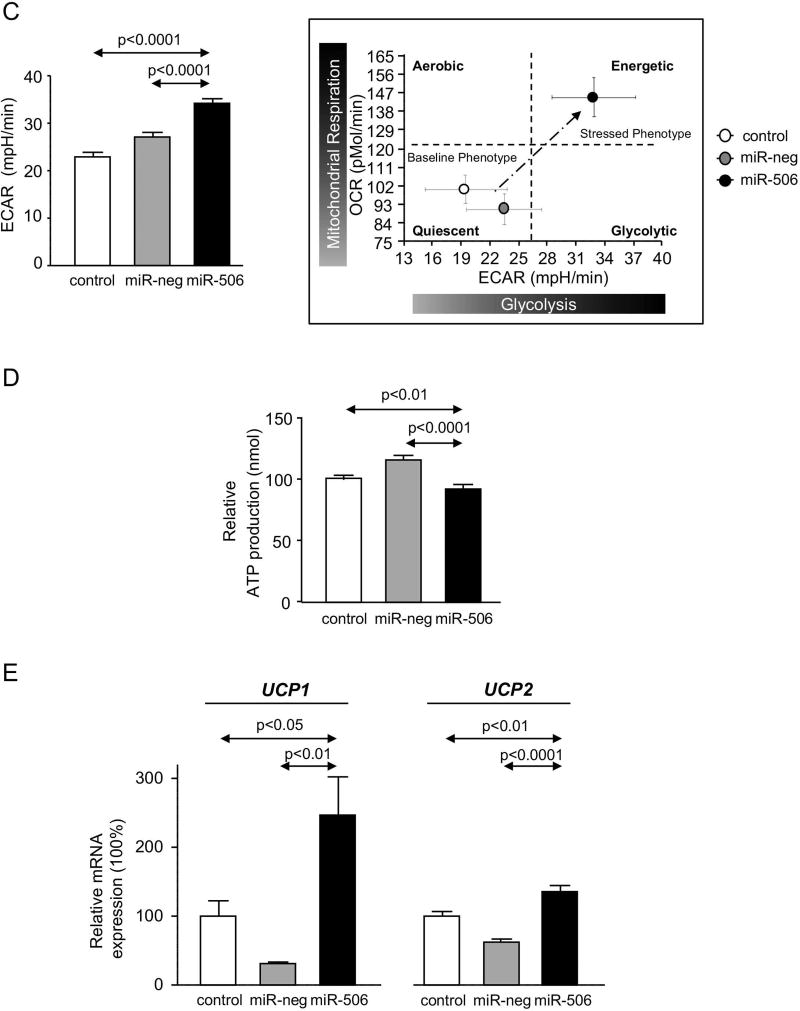
Since most of the proteins found dysregulated in cholangiocytes under miR-506 overexpression participate in metabolic processes (Figures 2C and D), mitochondrial metabolism was investigated in detail. Hence, oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were monitored upon sequential treatment with mitochondrial inhibitors in H69, H69-miR-neg and H69-miR-506 cells by using a Seahorse XF96 Extracellular Flux Analyzer (Figure 5A). MiR-506 altered mitochondrial energetic metabolism in cholangiocytes characterized by increased baseline respiration, maximal respiration, ATP-linked respiration and non-mitochondrial respiration compared to controls (Figure 5B). In addition, H69-miR-506 cells showed increased ECAR, glycolysis and oxidative phosphorylation (OXPHOS), and a more energetic phenotype compared to controls (Figure 5C). Notably, all these functional events in H69-miR-506 cells were also associated with increased proton leak compared to controls (Figure 5B), indicating higher uncoupling of mitochondrial ATP production from respiration in H69-miR-506 cells that results in overall decreased ATP production (Figure 5D). The increased uncoupled respiration in H69-miR-506 cells was confirmed by the upregulation of the mitochondrial uncoupling proteins UCP1 and UCP2 gene expression in H69-miR-506 cells compared to controls (Figure 5E). These functional data were also supported by the fact that different proteins involved in the mitochondrial energetic metabolism were found altered by proteomic analysis in cholangiocytes under miR-506 overexpression, particularly proteins involved in the mitochondrial energetic metabolism.
Figure 5. Cholangiocytes overexpressing miR-506 show increased mitochondrial metabolism.
(A) Representative “oxygen consumption rate” (OCR) of H69, H69-miR-neg and H69-miR-506 cells during mitochondrial stress test analyzed by “Seahorse technology”. (B) Metabolic parameters calculated upon OCR measurements. (C) Bar-graph showing “extracellular acidification rate” (ECAR) during mitochondrial stress test and representation of OCR vs ECAR for determining the metabolic-switch. Seahorse data corresponds to n=22–30 wells in each group. (D) ATP production rate (n=6). (E) UCP1 and UCP2 mRNA expression relative to GAPDH (n=6).
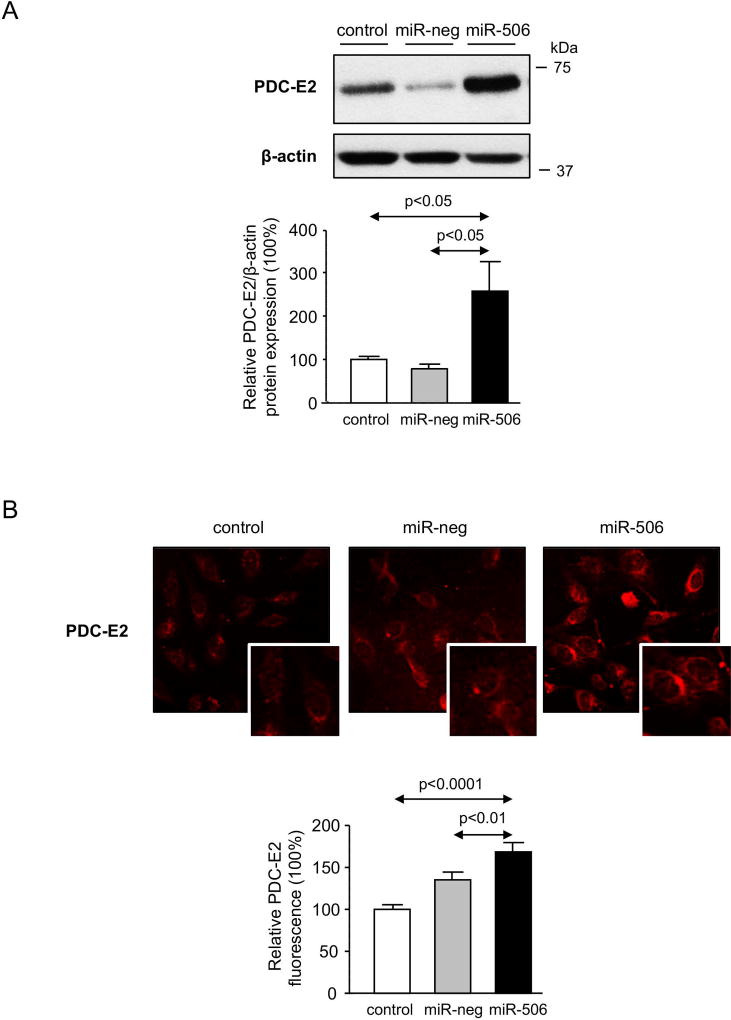
Relevance of miR-506 on PDC-E2 expression in cholangiocytes and on immune regulation
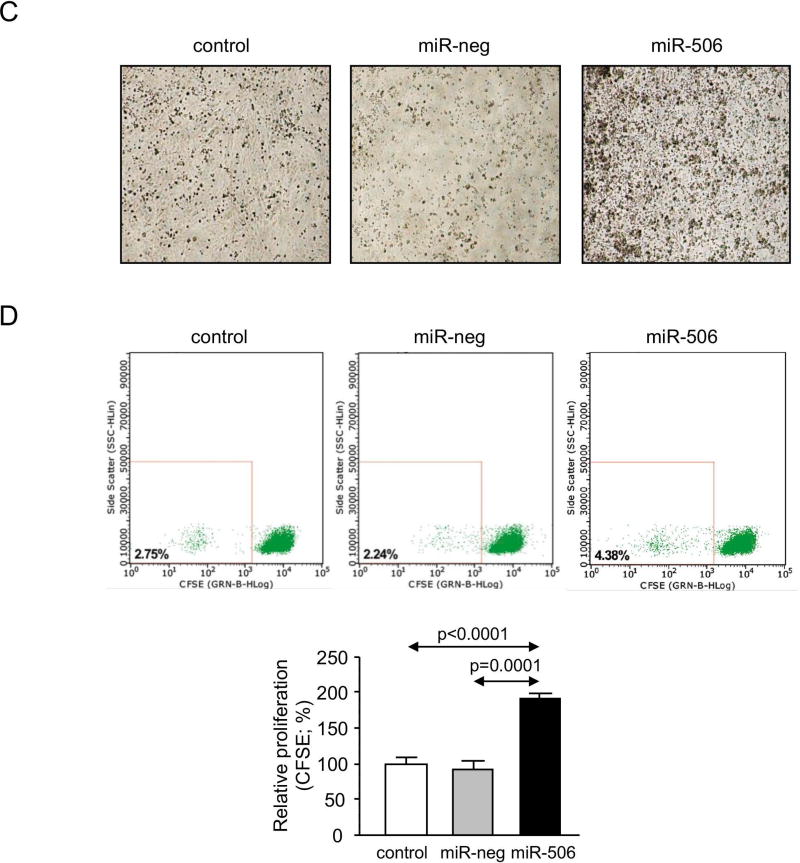
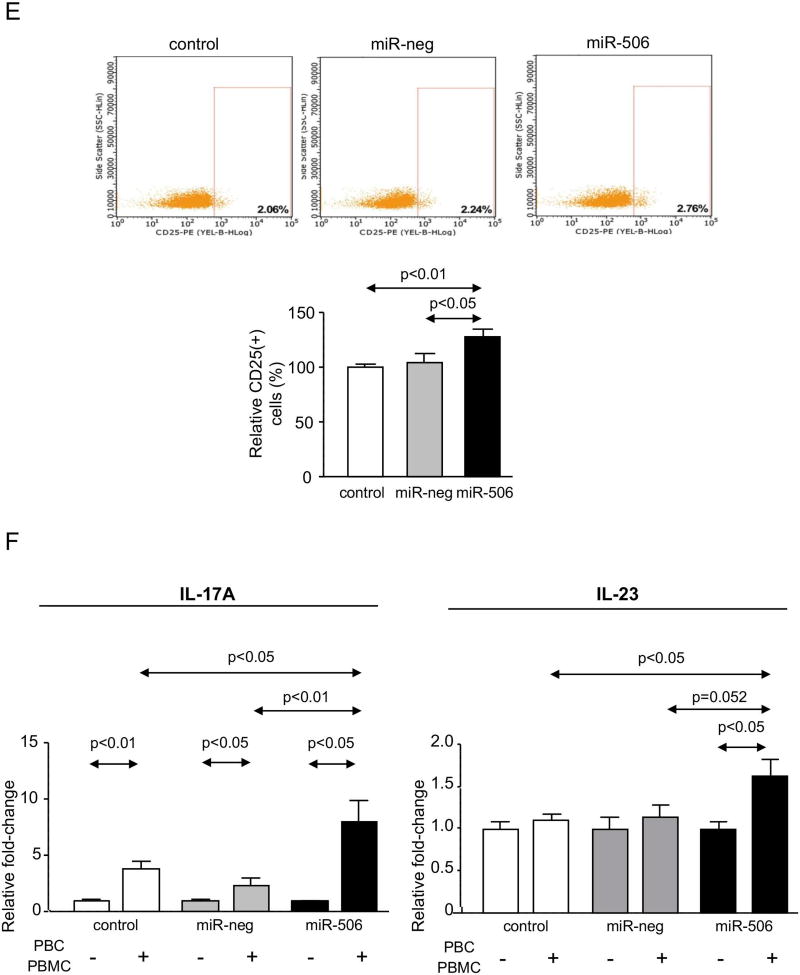
PBC patients are mostly characterized (~95%) by spontaneous development of AMA specific against PDC-E2, as a consequence of the overexpression and mislocalization of PDC-E2 in cholangiocytes, resulting in autoimmune phenomena.(24) Here, we found that H69-miR-506 cells under baseline condition present PDC-E2 overexpression (Figures 6A and B), which was found located in both cytoplasm and plasma membrane compared to control conditions, where PDC-E2 was mainly localized into the cytoplasm (Figure 6B). Next, co-cultures of H69, H69-miR-neg or H69-miR-506 cells together with PBC peripheral blood mononuclear cells (PBMCs) were carried out and the proliferation and activation of immunocytes were studied. Co-cultures of H69-miR-506 cholangiocytes together with PBMCs from a PBC patient induced the generation of higher number and size of lymphocyte-aggregates compared to control conditions (Figure 6C). Notably, these lymphocyte-aggregates were characterized by increased proliferation (CFSE assay by flow-cytometry; Figure 6D) and activation (CD25 marker by flow-cytometry; Figure 6E) of PBC PBMCs compared to controls. Likewise, co-culture of H69-miR-506 cholangiocytes together with PBMCs from a normal human donor also increased the proliferation and activation of PBMCs compared to controls (Supplementary Figure 8). The levels of cytokines involved in the immune response of PBC patients were also evaluated in the supernatant of the cells cultured alone or co-cultured with PBMCs from PBC patients. When co-cultured, H69-miR-506 cells showed higher levels of both IL17A and IL23 compared to controls (Figure 6F).
Figure 6. MiR-506 induces PDC-E2 overexpression in cholangiocytes and promotes immune activation.
(A) Representative immunoblot showing PDC-E2 protein overexpression in H69-miR-506 cells compared to controls. β-actin was used as housekeeping control. Bar-graph shows quantification (n=6). (B) Representative Immunofluorescent microscopy images showing PDC-E2 protein expression and location, and corresponding fluorescence quantification (n=40–54 cells for each group). (C–F) H69, H69-miR-neg and H69-miR-506 co-culture with PBMCs from a PBC patient was established (n=5). (C) Representative microscope images of the different co-culture conditions. PBMCs under co-culture were evaluated by (D) proliferation rate analysis with CFSE staining and (E) the activation by CD25 staining. Representative histograms and dot-blots are shown for each cell line co-cultured. (F) Levels of IL-17A and IL-23 in the supernatant of H69, H69-miR-neg and H69-miR-506 co-cultured with PBC PBMCs.
Discussion
The key findings reported here are related to the regulatory mechanisms of miR-506 expression in human cholangiocytes as well as to the role of miR-506 in cholangiocyte pathophysiology and immune activation. Our data indicate that: (i) miR-506 gene promoter activity is stimulated in cholangiocytes by different pro-inflammatory cytokines, but not by bile acids, estrogens or growth factors; (ii) stable overexpression of miR-506 in cholangiocytes inhibited AE2 protein expression and dysregulated the proteomic profile of the cells; (iii) miR-506 inhibited the expression of biliary and epithelial markers in cholangiocytes and promoted the expression of mesenchymal, pro-inflammatory, pro-fibrotic and senescence genes. This altered phenotype resulted in decreased cell proliferation, adhesion and migration; (iv) miR-506 stimulated ROS, ER stress and DNA damage in cholangiocytes, and sensitized the cells against toxic bile acid-induced apoptosis; (v) miR-506 increased the mitochondrial metabolism and OXPHOS associated with uncoupling of ATP production from mitochondrial respiration. These mitochondrial events were also associated with overexpression and mislocalization of PDC-E2; and (vi) miR-506 in cholangiocytes promoted the proliferation and activation of PBC lymphocytes. Our data are consistent with the notion that miR-506 is a key player in the pathogenesis of PBC and a potential target for therapy.
PBC is characterized by dysregulation of the miR expression profile in both liver and PBMCs,(25, 26) but their functional relevance still remains mostly unknown. In this regard, we previously found that miR-506 is exclusively expressed in the bile duct epithelial cells of PBC livers and its expression is increased compared to the bile duct cells of normal controls.(8) In cholangiocytes, miR-506 directly targets both AE2(8) and Ins3PR3(12) leading to cholestasis. However, the regulation of miR-506 expression and its role in cholangiocyte pathophysiology and immune regulation remain unknown. In the current report, the evaluation of different lengths of miR-506 promoter indicate that the full-length ~3 kb region of miR-506 promoter is required for its stimulation by pro-inflammatory cytokines found overexpressed in PBC livers such as IL8, IL12, IL17, IL18 and TNFα. These pro-inflammatory cytokines are involved in PBC immune response modulation and are associated with disease progression.(18, 29–32) Thus, in PBC patients, the cytokine profile in serum and liver samples suggests activation and liver recruitment of T-helper (Th)1 and Th17 cells.(30) lL12 and IL23, which are produced by antigen presenting cells, are responsible for promoting Th1 and Th17 immune responses, respectively. IL12 primarily promotes the differentiation of Th0 to Th1 cells (which are known to produce IFNγ, IL18 and TNFα), whereas IL23 is implicated in the differentiation of Th0 to Th17 that induces IL17 secretion.(33, 34) In contrast, other pro-inflammatory or pro-fibrotic cytokines, as well as estrogens –believed to participate in the pathogenesis of this disease that mainly affects middle-aged women–, growth factors, immunosuppresors, and choleretic but potentially cytotoxic (i.e. CA) or hypercholeretic and hepatoprotective (i.e. UDCA and TUDCA) bile acids did not show any effect on miR-506 expression. These data indicate that the full-length promoter sequence contains essential regulatory elements for the specific regulation of miR-506 expression in cholangiocytes under baseline and stimulated conditions, and highlight the importance of specific pro-inflammatory cytokines in the promotion of miR-506 expression.
We further evaluated the role of miR-506 in cholangiocyte pathophysiology. Experimental overexpression of miR-506 in cholangiocytes induced the dysregulation of proteins involved in fundamental biological processes. In particular, the expression of the mitochondrial proteins OPA1, ATP5H and CAPN1 was further validated by immunoblotting as downregulated (OPA1 and ATP5H) or upregulated (CAPN1) compared to controls. These abnormal expressions of OPA1, ATP5H and CAPN1 have been previously described to be involved in the generation of ER stress and/or ROS.(35–37) Additionally, miR-506 decreased the gene expression of biliary/epithelial markers and upregulated mesenchymal, pro-inflammatory and pro-fibrotic genes in cholangiocytes. In line with this, miR-506 inhibited cholangiocyte proliferation, adhesion and migration. These results mimic the phenotype of PBC cholangiocytes, which are characterized by dedifferentiation, inflammation and a pro-fibrogenic phenotype.(34) Bile ducts in PBC livers show senescence, DNA damage and ER stress.(19, 38, 39) In agreement with these PBC features, our data indicate that miR-506 induces cell senescence by increasing p21 expression and stimulates cellular stress by increasing ROS levels, ER stress and DNA damage; this altered phenotype did not result in baseline cholangiocyte cell death but sensitized cells against the caspase-3-dependent apoptosis induced by toxic bile acids. In contrast, the necrotic cell death pathway “necroptosis”, which was reported to be active in hepatocytes of an acute cholestatic animal model and PBC patients,(40) did not have any role on the bile acid-induced cholangiocyte death. The pro-apoptotic effect of miR-506 may be related, to a certain degree, to its direct targeting of AE2 mRNA, as experimental downregulation of AE2 in cholangiocytes has been shown to favor the bile salt-induced apoptosis (BSIA).(41) Moreover, GCDC has been described to reduce AE2 expression in biliary epithelial cells by inducing ROS.(42) These data pointed out the relevant role of miR-506 regulating the so called “AE2-related biliary bicarbonate umbrella”. AE2 downregulation impairs this protective barrier resulting in increased intracellular pH, accumulated toxic apolar hydrophobic bile acids and cell apoptosis.(43) All these PBC-like features observed in miR-506 overexpressing cholangiocytes may be responsible, at least partially, for the progressive ductopenia characteristic of the patients.(38)
The role of miR-506 was also evaluated on the mitochondrial metabolic activity and immune activation. Mitochondrial abnormalities are involved in the pathogenesis of PBC. Toxic bile acid accumulation during cholestasis leads to mitochondrial dysfunction through oxidative stress.(44) Our data showed that overexpression of miR-506 in cholangiocytes led to altered mitochondrial energetic metabolism characterized by increased oxygen consumption and glycolysis but also by increased proton leak, indicating a rise of uncoupling respiration. This increase in uncoupling could be in line with the increase in ROS production and perhaps with DNA damage and ER stress. Of note, miR-506-dependent mitochondrial dysfunction in cholangiocytes was also associated with PDC-E2 overexpression, a typical PBC feature that may promote, at least partially, the immunogenicity of cholangiocytes.(34) Therefore, we evaluated the capacity of miR-506 in cholangiocytes to modulate PBC lymphocytes under co-culture. Interestingly, miR-506 overexpressing cholangiocytes promoted the proliferation and activation of PBC PBMCs compared to cholangiocytes under control conditions. In addition, co-cultures of H69-miR-506 cells and PBMC increased both IL17 and IL23 levels, important cytokines in Th1 and Th17 immune responses and in PBC, as these pro-inflammatory cytokines are overexpressed in peripheral blood of PBC patients.(31) Of note, miR-506 overexpressing cholangiocytes also stimulated the proliferation and activation of PBMCs from a normal human donor compared to cholangiocytes under control conditions, suggesting that upregulation of miR-506 in PBC cholangiocytes may be an important cause of immune-activation. Therefore, PBC cholangiocytes appear not to be mere targets of an altered immune system and may be crucial in the promotion of immune reactions. These data are consistent with previous reports indicating that PBC cholangiocytes are characterized by overexpression and aberrant location, into the plasma membrane, of mitochondrial PDC-E2. Additionally, PBC cholangiocytes present increased apoptosis (20, 21) and immunologically active PDC-E2 due to the lack of glutathiolation,(45) which may be present in apoptotic bodies (known as apotopes).(45, 46) These two mechanisms of aberrant PDC-E2 presentation may be the base of the development of AMA and further promotion of autoimmunity against cholangiocytes.
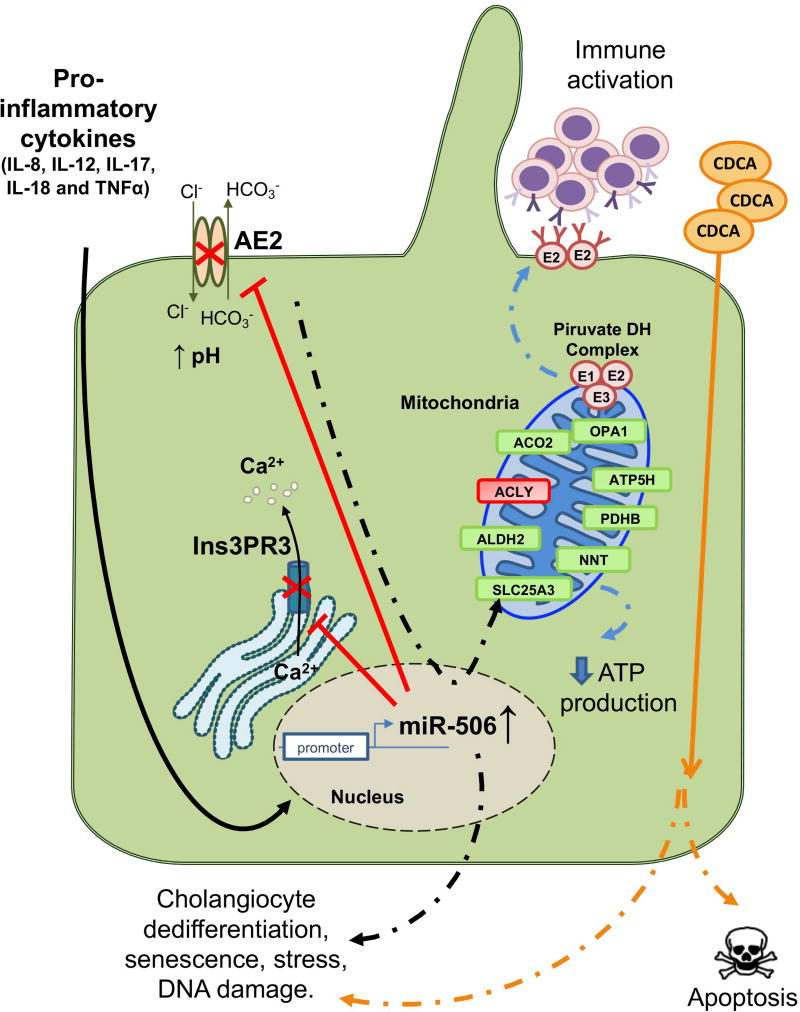
In summary, this study provides novel insights of the important role of miR-506 in the pathogenesis of PBC. Different pro-inflammatory cytokines found overexpressed in PBC livers promote the upregulation of miR-506 expression in cholangiocytes, leading to the development of PBC-like features such as cell dedifferentiation, stress, predisposition to bile-salt induced apoptosis, alterations in mitochondrial function and PDC-E2 overexpression, which finally result in PBC immune activation (Figure 7). These effects are mediated, to a certain degree, by direct targeting of miR-506 to both AE2 and Ins3PR3, which play key roles in the maintenance of the biliary phenotype. However, the role of miR-506 directly regulating the expression of other genes must also be considered and determined in future studies. MiR-506 represents a key player in the pathophysiology of PBC cholangiocytes and a potential target for therapy.
Figure 7. Working model.
Pro-inflammatory cytokines such as IL-8, IL-12, IL-17, IL-18 and TNFα stimulate the promoter activity of miR-506 gene. Overexpression of miR-506 in cholangiocytes inhibits AE2 and Ins3PR3 expression and activities, resulting in altered intracellular pH and Ca2+ concentration. MiR-506 leads to altered mitochondrial energetic metabolism associated with altered expression of proteins involved in such process; the resultant mitochondrial energetic metabolism fails and results in decreased ATP production and in overexpression and mislocalization of PDC-E2, leading to immune activation. MiR-506 decreases the expression of biliary and epithelial markers in cholangiocytes and increases the expression of mesenchymal, inflammatory and senescence genes, impairing cell proliferation, adhesion and migration, and stimulating ROS, ER stress and DNA damage. MiR-506 also sensitizes cholangiocytes against toxic bile acid-induced apoptosis.
Supplementary Material
Acknowledgments
Authors thank Dr. Maider Muñoz for her help with the co-culture studies and Dr. Ana Aiastui (cell culture platform of the Biodonostia Health Research Institute) for the support with the Seahorse studies.
Grant Support: Spanish Ministries of Economy and Competitiveness [J.M. Banales (FIS PI12/00380, FIS PI15/01132 and Miguel Servet Program CON14/00129); M.J. Perugorria (FIS PI14/00399); J.J.G. Marin (FIS PI16/00598 and SAF2013-40620-R)] cofinanced by “Fondo Europeo de Desarrollo Regional” (FEDER); “Instituto de Salud Carlos III” [CIBERehd: J.M. Banales, L. Bujanda and J.J.G. Marin (EHD15PI05)], Spain; “Junta de Castilla y Leon” (J.J.G. Marin: SA015U13 and BIO/SA52/15); “Diputación Foral Gipuzkoa” (L. Bujanda: DFG14/007; J.M. Banales: DFG15/010, DFG16/004), Departments of Industry, Tourism, Trade and Health of the Basque Country (L. Bujanda: 2013111173) and BIOEF (Basque Foundation for Innovation and Health Research: EiTB Maratoia BIO15/CA/016/BD to J.M. Banales); NIH Grant (M. Ananthanarayanan: 1R56 DK099470-01). J.M. Banales and O. Erice were funded by the “Asociación Española Contra el Cancer (AECC)” and A. Santos-Laso by the Basque Government. V. Torrado is supported by “Fundación Vasca de Innovación e Investigación Sanitarias” (BIOEF: BIO15/CA/052), Department of Health of the Basque Government and AECC-Bizkaia. A. Carracedo is supported by Ramón y Cajal award, the Basque Department of Industry, Tourism and Trade (Etortek), ISCIII (PI10/01484, PI13/00031), FERO VIII Fellowship, BBVA foundation, ///MINECO (SAF2016-79381-R; AEI/FEDER, EU) and the European Research Council Starting Grant (336343). CIBERONC was co-funded with FEDER funds. The Proteomics unit of Navarrabiomed is a member of Proteored, PRB2-ISCIII, and is supported by grant PT13/0001, of the PE I+D+I 2013–2016 funded by ISCIII and FEDER.
Abbreviations
- 7AAD
7-aminoactinomycin D
- AE2/SLC4A2
Cl−/HCO3− exchanger 2 (anion exchanger 2)
- αSMA
alpha smooth muscle actin
- AMA
anti-mitochondrial auto-antibodies
- ATF6
activating transcription factor 6
- ATP5H
ATP synthase subunit D
- CA
cholic acid
- Ca2+i
intracellular calcium
- CAPN1
calpain 1
- CDCA
chenodeoxycholic acid
- CFSE
carboxyfluorescein succinimidyl ester
- CCA
cholangiocarcinoma
- CHOP
CCAAT-enhancer-binding protein homologous protein
- CK7
cytokeratin 7
- Cl−/HCO3−
chlorine bicarbonate exchange
- DEX
dexamethasone
- DHE
dihydroethidium
- DMEM/F12
dulbecco's modified eagle medium nutrient mixture F-12
- DMSO
dimethyl sulfoxyde
- ECAR
extracellular acidification rate
- EGF
epidermal growth factor
- ER
endoplasmic reticulum
- FCCP
carbonyl cyanide-4-(trifluoromethoxy) phenylhydrazone
- FDA
food and drug administration
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GCDCA
glycochenodeoxycholic acid
- HCC
hepatocellular carcinoma
- HRP
horseradish peroxidase
- IFNγ
interferon gamma
- IL
interleukin
- InsP3R3
type III inositol 1,4,5-trisphosphate receptor
- IRE1
inositol-requiring enzyme 1
- miR
microRNA
- OCR
oxygen consumption rate
- OPA1
dynamin-like 120 kDa protein
- OXPHOS
oxidative phosphorylation
- PBC
primary biliary cholangitis
- PBMC
peripheral blood mononuclear cell
- PDC-E2
pyruvate dehydrogenase complex E2
- PDGFB
platelet-derived growth factor subunit B
- PERK
PRKR-like ER kinase
- PHA
phytohaemagglutinin
- qPCR
quantitative polymerase chain reaction
- RPMI
roswell park memorial institute medium
- ROS
reactive oxygen species
- RT-PCR
reverse transcription polymerase chain reaction
- SEM
standard error of mean
- TCDCA
taurochenodeoxycholic acid
- TGFβ1
transforming growth factor-beta 1
- TGFβ2
transforming growth factor-beta 2
- TNFα
tumor necrosis factor alpha
- TUDCA
tauroursodeoxycholic acid
- UCP
uncoupling protein
- UDCA
ursodeoxycholic acid
- VEGF
vascular endothelial growth factor
- XBP1
X-box binding protein 1
- ZO1
zonula occludens 1
- γH2AX
phosphorylated histone H2A variant X protein
Footnotes
Conflict of interest: authors disclose no conflicts.
Author Contributions:
OE, PM-G, JV, MJP, MGF-B, ES, AS-L, AA, RJA, JF-I, ES, VT, AC, MA, MM, JP, UB, ROE, NFL, LB, JJGM, JMB: study concept and design, analysis and interpretation of data, drafting of the manuscript. OE, PM-G, JV, MJP, MGF-B, ES, AS-L, AA, JF-I, ES, VT, AC, MA, JJGM, JMB: acquisition of data. OE, PM-G, JV, MJP, MGF-B, ES, AS-L, AA, JF-I, ES, VT, AC, MA, JJGM, JMB: statistical analysis. LB, JJGM, JMB: obtained funding.
References
- 1.Beuers U, Gershwin ME, Gish RG, Invernizzi P, Jones DE, Lindor K, et al. Changing nomenclature for PBC: from 'cirrhosis' to 'cholangitis'. Gastroenterology. 2015;149:1627–1629. doi: 10.1053/j.gastro.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261–1273. doi: 10.1056/NEJMra043898. [DOI] [PubMed] [Google Scholar]
- 3.Melero S, Spirli C, Zsembery A, Medina JF, Joplin RE, Duner E, et al. Defective regulation of cholangiocyte Cl-/HCO3(−) and Na+/H+ exchanger activities in primary biliary cirrhosis. Hepatology. 2002;35:1513–1521. doi: 10.1053/jhep.2002.33634. [DOI] [PubMed] [Google Scholar]
- 4.Prieto J, Garcia N, Marti-Climent JM, Penuelas I, Richter JA, Medina JF. Assessment of biliary bicarbonate secretion in humans by positron emission tomography. Gastroenterology. 1999;117:167–172. doi: 10.1016/s0016-5085(99)70564-0. [DOI] [PubMed] [Google Scholar]
- 5.Banales JM, Arenas F, Rodriguez-Ortigosa CM, Saez E, Uriarte I, Doctor RB, et al. Bicarbonate-rich choleresis induced by secretin in normal rat is taurocholate-dependent and involves AE2 anion exchanger. Hepatology. 2006;43:266–275. doi: 10.1002/hep.21042. [DOI] [PubMed] [Google Scholar]
- 6.Arenas F, Hervias I, Uriz M, Joplin R, Prieto J, Medina JF. Combination of ursodeoxycholic acid and glucocorticoids upregulates the AE2 alternate promoter in human liver cells. J Clin Invest. 2008;118:695–709. doi: 10.1172/JCI33156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez-Anso E, Castillo JE, Diez J, Medina JF, Prieto J. Immunohistochemical detection of chloride/bicarbonate anion exchangers in human liver. Hepatology. 1994;19:1400–1406. [PubMed] [Google Scholar]
- 8.Banales JM, Saez E, Uriz M, Sarvide S, Urribarri AD, Splinter P, et al. Up-regulation of microRNA 506 leads to decreased Cl-/HCO3- anion exchanger 2 expression in biliary epithelium of patients with primary biliary cirrhosis. Hepatology. 2012;56:687–697. doi: 10.1002/hep.25691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salas JT, Banales JM, Sarvide S, Recalde S, Ferrer A, Uriarte I, et al. Ae2a,b-deficient mice develop antimitochondrial antibodies and other features resembling primary biliary cirrhosis. Gastroenterology. 2008;134:1482–1493. doi: 10.1053/j.gastro.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 10.Minagawa N, Nagata J, Shibao K, Masyuk AI, Gomes DA, Rodrigues MA, et al. Cyclic AMP regulates bicarbonate secretion in cholangiocytes through release of ATP into bile. Gastroenterology. 2007;133:1592–1602. doi: 10.1053/j.gastro.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirata K, Nathanson MH. Bile duct epithelia regulate biliary bicarbonate excretion in normal rat liver. Gastroenterology. 2001;121:396–406. doi: 10.1053/gast.2001.26280. [DOI] [PubMed] [Google Scholar]
- 12.Ananthanarayanan M, Banales JM, Guerra MT, Spirli C, Munoz-Garrido P, Mitchell-Richards K, et al. Post-translational regulation of the type III inositol 1,4,5-trisphosphate receptor by miRNA-506. J Biol Chem. 2015;290:184–196. doi: 10.1074/jbc.M114.587030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salter KD, Roman RM, LaRusso NR, Fitz JG, Doctor RB. Modified culture conditions enhance expression of differentiated phenotypic properties of normal rat cholangiocytes. Lab Invest. 2000;80:1775–1778. doi: 10.1038/labinvest.3780187. [DOI] [PubMed] [Google Scholar]
- 14.Unwin RD, Griffiths JR, Whetton AD. Simultaneous analysis of relative protein expression levels across multiple samples using iTRAQ isobaric tags with 2D nano LC-MS/MS. Nat Protoc. 2010;5:1574–1582. doi: 10.1038/nprot.2010.123. [DOI] [PubMed] [Google Scholar]
- 15.Zelaya MV, Perez-Valderrama E, de Morentin XM, Tunon T, Ferrer I, Luquin MR, et al. Olfactory bulb proteome dynamics during the progression of sporadic Alzheimer's disease: identification of common and distinct olfactory targets across Alzheimer-related co-pathologies. Oncotarget. 2015;6:39437–39456. doi: 10.18632/oncotarget.6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shilov IV, Seymour SL, Patel AA, Loboda A, Tang WH, Keating SP, et al. The Paragon Algorithm, a next generation search engine that uses sequence temperature values and feature probabilities to identify peptides from tandem mass spectra. Mol Cell Proteomics. 2007;6:1638–1655. doi: 10.1074/mcp.T600050-MCP200. [DOI] [PubMed] [Google Scholar]
- 17.McDaniel K, Meng F, Wu N, Sato K, Venter J, Bernuzzi F, et al. Forkhead box A2 regulates biliary heterogeneity and senescence during cholestatic liver injury in micedouble dagger. Hepatology. 2017;65:544–559. doi: 10.1002/hep.28831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang Q, Chu S, Yin X, Yu X, Kang C, Li X, et al. Interleukin-17A-Induced Epithelial-Mesenchymal Transition of Human Intrahepatic Biliary Epithelial Cells: Implications for Primary Biliary Cirrhosis. Tohoku J Exp Med. 2016;240:269–275. doi: 10.1620/tjem.240.269. [DOI] [PubMed] [Google Scholar]
- 19.Sasaki M, Yoshimura-Miyakoshi M, Sato Y, Nakanuma Y. A possible involvement of endoplasmic reticulum stress in biliary epithelial autophagy and senescence in primary biliary cirrhosis. J Gastroenterol. 2015;50:984–995. doi: 10.1007/s00535-014-1033-0. [DOI] [PubMed] [Google Scholar]
- 20.Harada K, Ozaki S, Gershwin ME, Nakanuma Y. Enhanced apoptosis relates to bile duct loss in primary biliary cirrhosis. Hepatology. 1997;26:1399–1405. doi: 10.1002/hep.510260604. [DOI] [PubMed] [Google Scholar]
- 21.Koga H, Sakisaka S, Ohishi M, Sata M, Tanikawa K. Nuclear DNA fragmentation and expression of Bcl-2 in primary biliary cirrhosis. Hepatology. 1997;25:1077–1084. doi: 10.1002/hep.510250505. [DOI] [PubMed] [Google Scholar]
- 22.Hohenester S, Wenniger LM, Paulusma CC, van Vliet SJ, Jefferson DM, Elferink RP, et al. A biliary HCO3- umbrella constitutes a protective mechanism against bile acid-induced injury in human cholangiocytes. Hepatology. 2012;55:173–183. doi: 10.1002/hep.24691. [DOI] [PubMed] [Google Scholar]
- 23.Hirata K, Dufour JF, Shibao K, Knickelbein R, O'Neill AF, Bode HP, et al. Regulation of Ca(2+) signaling in rat bile duct epithelia by inositol 1,4,5-trisphosphate receptor isoforms. Hepatology. 2002;36:284–296. doi: 10.1053/jhep.2002.34432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ali AH, Tabibian JH, Carey EJ, Lindor KD. Emerging drugs for the treatment of Primary Biliary Cholangitis. Expert Opin Emerg Drugs. 2016;21:39–56. doi: 10.1517/14728214.2016.1150999. [DOI] [PubMed] [Google Scholar]
- 25.Padgett KA, Lan RY, Leung PC, Lleo A, Dawson K, Pfeiff J, et al. Primary biliary cirrhosis is associated with altered hepatic microRNA expression. J Autoimmun. 2009;32:246–253. doi: 10.1016/j.jaut.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qin B, Huang F, Liang Y, Yang Z, Zhong R. Analysis of altered microRNA expression profiles in peripheral blood mononuclear cells from patients with primary biliary cirrhosis. J Gastroenterol Hepatol. 2013;28:543–550. doi: 10.1111/jgh.12040. [DOI] [PubMed] [Google Scholar]
- 27.Medina JF, Martinez A, Vazquez JJ, Prieto J. Decreased anion exchanger 2 immunoreactivity in the liver of patients with primary biliary cirrhosis. Hepatology. 1997;25:12–17. doi: 10.1002/hep.510250104. [DOI] [PubMed] [Google Scholar]
- 28.Shibao K, Hirata K, Robert ME, Nathanson MH. Loss of inositol 1,4,5-trisphosphate receptors from bile duct epithelia is a common event in cholestasis. Gastroenterology. 2003;125:1175–1187. doi: 10.1016/s0016-5085(03)01201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chuang YH, Lian ZX, Tsuneyama K, Chiang BL, Ansari AA, Coppel RL, et al. Increased killing activity and decreased cytokine production in NK cells in patients with primary biliary cirrhosis. J Autoimmun. 2006;26:232–240. doi: 10.1016/j.jaut.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Yang CY, Ma X, Tsuneyama K, Huang S, Takahashi T, Chalasani NP, et al. IL-12/Th1 and IL-23/Th17 biliary microenvironment in primary biliary cirrhosis: implications for therapy. Hepatology. 2014;59:1944–1953. doi: 10.1002/hep.26979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qian C, Jiang T, Zhang W, Ren C, Wang Q, Qin Q, et al. Increased IL-23 and IL-17 expression by peripheral blood cells of patients with primary biliary cirrhosis. Cytokine. 2013;64:172–180. doi: 10.1016/j.cyto.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Yamano T, Higashi T, Nouso K, Nakatsukasa H, Kariyama K, Yumoto E, et al. Serum interferon-gamma-inducing factor/IL-18 levels in primary biliary cirrhosis. Clin Exp Immunol. 2000;122:227–231. doi: 10.1046/j.1365-2249.2000.01356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kikly K, Liu L, Na S, Sedgwick JD. The IL-23/Th(17) axis: therapeutic targets for autoimmune inflammation. Curr Opin Immunol. 2006;18:670–675. doi: 10.1016/j.coi.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Dyson JK, Hirschfield GM, Adams DH, Beuers U, Mann DA, Lindor KD, et al. Novel therapeutic targets in primary biliary cirrhosis. Nat Rev Gastroenterol Hepatol. 2015;12:147–158. doi: 10.1038/nrgastro.2015.12. [DOI] [PubMed] [Google Scholar]
- 35.Tezze C, Romanello V, Desbats MA, Fadini GP, Albiero M, Favaro G, et al. Age-Associated Loss of OPA1 in Muscle Impacts Muscle Mass, Metabolic Homeostasis, Systemic Inflammation, and Epithelial Senescence. Cell Metab. 2017;25:1374–1389. e1376. doi: 10.1016/j.cmet.2017.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Houstek J, Pickova A, Vojtiskova A, Mracek T, Pecina P, Jesina P. Mitochondrial diseases and genetic defects of ATP synthase. Biochim Biophys Acta. 2006;1757:1400–1405. doi: 10.1016/j.bbabio.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Ni R, Zheng D, Xiong S, Hill DJ, Sun T, Gardiner RB, et al. Mitochondrial Calpain-1 Disrupts ATP Synthase and Induces Superoxide Generation in Type 1 Diabetic Hearts: A Novel Mechanism Contributing to Diabetic Cardiomyopathy. Diabetes. 2016;65:255–268. doi: 10.2337/db15-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sasaki M, Ikeda H, Yamaguchi J, Nakada S, Nakanuma Y. Telomere shortening in the damaged small bile ducts in primary biliary cirrhosis reflects ongoing cellular senescence. Hepatology. 2008;48:186–195. doi: 10.1002/hep.22348. [DOI] [PubMed] [Google Scholar]
- 39.Sasaki M, Ikeda H, Haga H, Manabe T, Nakanuma Y. Frequent cellular senescence in small bile ducts in primary biliary cirrhosis: a possible role in bile duct loss. J Pathol. 2005;205:451–459. doi: 10.1002/path.1729. [DOI] [PubMed] [Google Scholar]
- 40.Afonso MB, Rodrigues PM, Simao AL, Ofengeim D, Carvalho T, Amaral JD, et al. Activation of necroptosis in human and experimental cholestasis. Cell Death Dis. 2016;7:e2390. doi: 10.1038/cddis.2016.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang JC, Go S, de Waart DR, Munoz-Garrido P, Beuers U, Paulusma CC, et al. Soluble Adenylyl Cyclase Regulates Bile Salt-Induced Apoptosis in Human Cholangiocytes. Hepatology. 2016;64:522–534. doi: 10.1002/hep.28550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hisamoto S, Shimoda S, Harada K, Iwasaka S, Onohara S, Chong Y, et al. Hydrophobic bile acids suppress expression of AE2 in biliary epithelial cells and induce bile duct inflammation in primary biliary cholangitis. J Autoimmun. 2016 doi: 10.1016/j.jaut.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 43.Beuers U, Hohenester S, de Buy Wenniger LJ, Kremer AE, Jansen PL, Elferink RP. The biliary HCO(3)(−) umbrella: a unifying hypothesis on pathogenetic and therapeutic aspects of fibrosing cholangiopathies. Hepatology. 2010;52:1489–1496. doi: 10.1002/hep.23810. [DOI] [PubMed] [Google Scholar]
- 44.Perez MJ, Briz O. Bile-acid-induced cell injury and protection. World J Gastroenterol. 2009;15:1677–1689. doi: 10.3748/wjg.15.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Odin JA, Huebert RC, Casciola-Rosen L, LaRusso NF, Rosen A. Bcl-2-dependent oxidation of pyruvate dehydrogenase-E2, a primary biliary cirrhosis autoantigen, during apoptosis. J Clin Invest. 2001;108:223–232. doi: 10.1172/JCI10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lleo A, Selmi C, Invernizzi P, Podda M, Coppel RL, Mackay IR, et al. Apotopes and the biliary specificity of primary biliary cirrhosis. Hepatology. 2009;49:871–879. doi: 10.1002/hep.22736. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.