Abstract
Purpose
To compare the relationship between obesity markers Body Mass Index (BMI) and Back Fat Thickness (BFT) and oedema in the lumbo-sacral subcutaneous adipose tissue.
Patients and methods
A retrospective study was performed of consecutive Magnetic Resonance Imaging examinations on 149 adults (95 females and 54 males) scanned at 1.5T between October 1 and December 31, 2010. The extent of oedema was graded from 1 to 8 based on the number of involved anatomical segments on the Fat Sat sequence. A vertebra and the disc immediately inferior or any of the upper, middle or lower third of the sacrum was assigned 1 unit. BFT was measured superiorly at the upper border of L1 (BFT L1) and inferiorly at the lower border of L5 (BFT L5) on the T1 weighted image. BMI was computed at the time of the examination. The data were analysed using StatPlus 2009. The association between variables was evaluated using univariate and multivariate regression.
Results
68 patients (45.6%), 50 females (33.6%) and 18 males (12.0%) were found to have oedema. Weight (p = 0), BMI (p < 0.001), BFT L1 (p < 0.001), BFT L5 (p < 0.001) and age (p = .01) were significantly associated with oedema. On forward stepwise multiple regression significant independent variables predicting oedema were found to be BMI, BFT L1 and Age. ANOVA indicated that BMI explained 23.6% (F = 45.5, p = 0), BFT L1 22.7% (F = 43.2, p = 0) and age 4.7% (F = 7.4, p = 0.007) of the variance of oedema.
Conclusions
Obesity markers BMI and BFT L1 are significant independent variables predicting oedema. Oedema is predicted to a variable extent by fat at different sites. The oedema may be, in part, a consequence of obesity.
Keywords: Medicine, Medical imaging, Pathology, Physiology
1. Introduction
Oedema-like signal (for simplicity, the term “oedema” is used for the remainder of the text) has been noted in the subcutaneous fat of the lumbar region on MRI during routine scans of patients who are not known clinically to have fluid overload, but little has been published on this entity. Shi et al. reported that the oedema was associated with body weight. They also speculated on the role that obesity may play in producing the oedema but they did not evaluate the association with BMI [1]. Genu et al. found a significant link between the oedema and being overweight, older, being hospitalised and the maximum thickness of the lumbar fat; they, however, also did not assess the correlation with BMI [2]. The present authors have found that this oedema shows significant positive correlation with BMI with an Odds Ratio of 8.6 in the obese compared to the non-obese [3]. This significant association with obesity prompted the authors to seek to determine how another means of evaluating obesity, Back Fat Thickness (BFT), predicted oedema. BFT is used in the meat industry to assess the fat content of carcasses. BFT has been shown to have a genetic basis in cattle and genes for obesity in human beings are similar to those associated with BFT in pigs [4, 5]. BFT has been shown to be a good index of obesity in beagles but our search of the literature did not reveal any studies reviewing similar BFT measurements in humans [6].
Wallner-Liebmann et al. have assessed obesity using measurements of the subcutaneous fat but they did not measure BFT. They used an optical Lipometer device to measure subcutaneous adipose tissue topography (SAT-Top) which consists of 15 well-defined body sites distributed from neck to calf on the right side of the body. They reported that subcutaneous fat patterns are a better screening tool than BMI to characterize fatness in physically active young people [7]. Measurement of BFT is usually made by ultrasonography in animals. Excess body fat produces harmful effects by various mechanisms including thrombogenic, atherogenic, oncogenic, hemodynamic and neurohumoral pathways but several studies have shown that some overweight persons may have similar or even better cardiovascular outcomes than patients with normal weight [8, 9].
This unexpected finding is due, at least in part, to the fact that the size of particular fat depots vary in patients with the same BMI but different ethnicity [10]. Also, adipose tissue is not a single homogeneous entity; specific regional depots of adipose tissue have different biological functions [11, 12]. Individual adipose tissue compartments have stronger associations with physiological and pathological processes than does total adipose tissue mass [13]. In light of the utility of BFT in other species and the inhomogeneity of adipose tissue in human beings we thought it would be useful to compare how BMI and BFT at two sites, one in the middle of the back at L1 and the other inferiorly at L5, predicted the oedema.
2. Materials and method
This was a retrospective case control review. The study was granted exemption from review by the Ethics Board of the Faculty of Medical Sciences, University of the West Indies, Mona, Jamaica there being little risk to the patients.
2.1. Patient population
The computerized database of our MRI Unit was reviewed for all patients 18 years and older who underwent MRI of the lumbar spine during the October 1 to December 31, 2010. The images and patient data were retrieved. Patients were excluded if they had had recent back surgery or trauma, or a history of renal disease, cardiac disease or hepatic failure. Patients were also excluded if the image quality of the Fat Sat examination were not satisfactory or if the Fat Sat sequence were not done. The criteria were pre-established.
2.2. Imaging technique
Imaging was performed on a 1.5-T MR imaging unit (Signa; General Electric Medical Systems, Milwaukee, WI). The subcutaneous posterior lumbar soft- tissue oedema was evaluated on fat-saturated T2-weighted sagittal fast spin- echo images. The MR imaging parameters were as follows: echo-train length, 8; TR range/TE range for sagittal T2-weighted sequences, 3100-4000/80-90; TR/TE for sagittal T1-weighted sequences, 400/9. The following parameters were the same for all pulse sequences: section thickness, 4.0 mm; intersection gap, 1.0 mm; matrix, 512 × 192; and field of view, 30 × 30 cm.
2.3. Image analysis
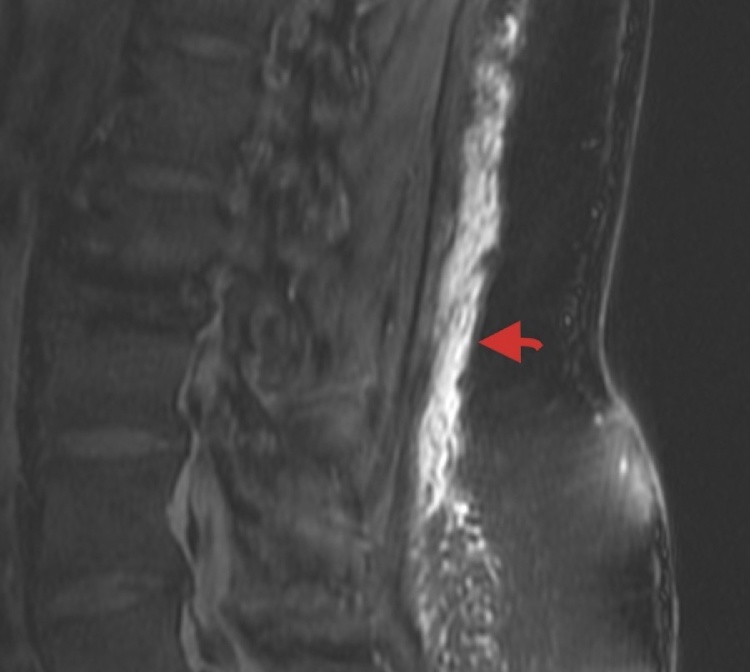
The lumbar MR images were retrospectively reviewed by one author (WW), consultant radiologist of 24years experience for the presence and extent of oedema in the subcutaneous fat on the sagittal T2 Fat Sat sequence. Fig. 1 The extent of oedema overlying the lumbar spine was graded with respect to the vertebral bodies and the inter-vertebral discs. Oedema overlying all or part of the combination of a single vertebral body and the disc immediately inferior to it was assigned a value of 1.
Fig. 1.
Sagittal Fat Sat MR image. Oedema in the lumbar subcutaneous fat. Arrow points to oedema.
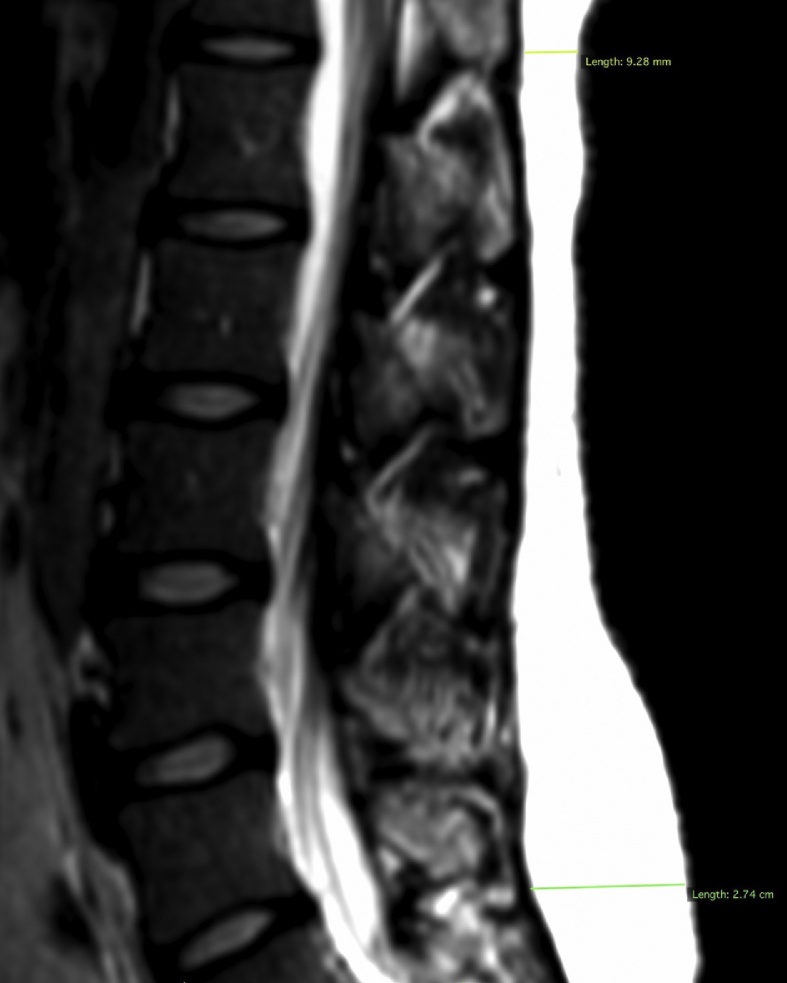
The extent of oedema overlying the sacrum was graded by dividing the sacrum into upper, middle and lower thirds. Each third was assigned a value of 1 Measurements of Back Fat Thickness were made on the sagittal T1 weighted image of the patient’s right side which provided the most complete view of the L5 vertebra. Measurements of the fat were made parallel to the inferior border of the image along a line which would pass through the postero-superior angle of the vertebral body at L1 and through the postero-inferior angle of L5 (Fig. 2).
Fig. 2.
Sagittal T1 weighted MR image indicating sites for measuring BFT L1 and BFT L5.
2.4. Statistical analysis
Data were analyzed using StatPlus:mac 2009. T-tests were used to compare means. Linear, forward stepwise, ANOVA, Odd’s Ratio, McNemar and chi-squared test were done in evaluating the presence of oedema. Spearman’s rho was used to determine the correlation between BMI, BFT and age and the extent of the oedema. A value of P < 0.02 was considered statistically significant.
3. Results
169 examinations were performed on 65 males and 104 females. 20 examinations on 11 males and 9 females were excluded from the study. Of these, 11 patients had acute back injury. 3 patients were excluded because of a history of renal failure. The Fat Sat examination was inadequate in 3 patients and was not done in 1 patient. 1 patient was excluded because of a tumour involving the soft tissues of the back. The finding of oedema was equivocal in 1 patient. The indications for the examinations for patients included in the study were: lower back pain, lower back pain with nerve root compression, sciatica and possible secondaries.
149 patients, aged between 22years and 81years, consisting of 95 females (mean age: 46.6 (99% confidence interval (CI) 43.2–50.0) years and 54 males (mean age:46.8 (99% confidence interval (CI) 41.7–51.9) years were included in the study. The difference in the means was not significant p = 0.9. The values of BMI, BFT L1 and BFT L5 for the sample were: BMI: range 17.2–42.5 kg/m2, mean 27.4 (99% confidence interval (CI) 26.4–28.4) kg/m2. BFT L1: range 0.2–5.3 cm, mean 2.2 (99% confidence interval (CI) 1.9–2.4) cm and BFT L5: range 0.4–7.3 cm, mean 3.0 (99% confidence interval (CI) 2.7–3.3) cm. 68 patients, 50 females and 18 males were positive for oedema in the lumbar subcutaneous tissue.
The mean weight of patients without oedema was 72.8 (99% confidence interval (CI) 69.4–76.2) kg and that of patients with oedema 85.9 (99% confidence interval (CI) 80.7–91.2) kg, the difference in the means was significant p = 0 Patients with oedema had significantly larger means for BMI 30.3 (99% confidence interval (CI) 28.8–31.8) kg/m2 vs. 24.9 (99% confidence interval (CI) 24.0–25.9) kg/m2, p < 0.001), BFT L1 2.8 (99% confidence interval (CI) 2.5–3.1) cm vs. 1.6 (99% confidence interval (CI) 1.4–1.9) cm, p < 0.001, BFT L5 3.9 (99% confidence interval (CI) 3.5–4.3) cm vs. 2.3 (99% confidence interval (CI) 2.0–2.7) cm, p < 0.001) and age (49.9 (99% confidence interval (CI) 46.0–53.8) years vs. 43.9 (99% confidence interval (CI) 39.9–47.9) years, p = .01) than patients without oedema (Table 1). The Spearman’s rho correlation test showed that BFT L1 (rs = .55, n = 149, p = 0, BMI (rs = .51, n = 149, p = 0) and BFT L5 (rs = .47, n = 149, p = 0) had significant moderate positive correlation with oedema. Age (r = .22, n = 149, p = .005) had significant positive correlation but to a lesser extent than the other three parameters. The data comparing all males and females are in Table 2. Using a BMI of 30 kg/m2 to define obesity the Odds Ratio of oedema in the obese compared to the non-obese was 8.6. The chi-square and McNemar tests were significant, p = 0 and p < .001 respectively.
Table 1.
Characteristics of patients with and without edema in the subcutaneous fat of the lumbar region on MRI.
| Characteristics | Patients with edema (n = 68) | Patients without edema (n = 81) | p |
|---|---|---|---|
| Age (mean), years | 49.9 ± 13.6 | 43.9 ± 15.1 | P = .01 |
| Men | 18 (12.1%) | 36 (24.2%) | P = .056 |
| Women, n (%) | 50 (33.6%) | 45 (30.2%) | |
| Weight, kg | 85.9 ± 18.2 | 72.8 ± 12.9 | P = 0 |
| BMI, kg/m2 | 30.3 ± 5.1 | 24.9 ± 3.7 | P < .001 |
| BFT L1, cm | 2.8 ± 1.1 | 1.6 ± 0.9 | p < .001 |
| BFT L5, cm | 3.9 ± 1.4 | 2.3 ± 1.3 | P < .001 |
BMI = Body Mass Index, BFT L1 = Back Fat Thickness at superior surface L1.
BFT L5 = Back Fat Thickness at superior surface L5.
Table 2.
Age, BMI, BFT L1 and BFT L5 of all females and males.
| Characteristics | Females (n = 95) | Males (n = 54) | p |
|---|---|---|---|
| Age (mean), years | 46.6 ± 14.2 | 46.8 ± 15.5 | P = .9 |
| BMI, kg/m2 | 28.1 ± 5.1 | 26.3 ± 5.1 | P = .04 |
| BFT L1, cm | 2.4 ± 1.1 | 1.8 ± 1.0 | p = .001 |
| BFT L5, cm | 3.5 ± 1.4 | 2.2 ±1.5 | P = .0 |
BMI = Body Mass Index, BFT L1 = Back Fat Thickness at superior surface L1.
BFT L5 = Back Fat Thickness at superior surface L5.
On forward stepwise multiple regression of BMI, BFT L1, BFT L5, Age and Sex, significant independent variables predicting oedema were found to be BMI, BFT L1 and Age. BFT L5 and sex were not found to be significant independent predictors of oedema. ANOVA indicated that BMI explained 23.6% (F = 45.5, p = 0), BFT L1 22.7% (F = 43.2, p = 0) and age 4.7% (F = 7.4, p = 0.007) of the variance of oedema. Kappa for intra – observer difference was 0.71.
4. Discussion
The oedema observed in the lumbar subcutaneous fat is due to extra-cellular fluid. The most common source of the extra-cellular fluid is fluid overload but since this possibility was reduced by patient selection other aetiologies such as micro- vascular dysfunction or abnormalities in the pressures in the vessels must be entertained.
In view of this second possibility we thought it useful to use a measurement which captured the anatomical distribution of the oedema as this would allow appreciation of the extent of segmental change in the blood vessels. There are no established protocols for measuring of Back Fat Thickness in humans similar to those for animals in the meat industry so our choice of sites to perform measurements was somewhat arbitrary. We thought it reasonable to obtain measurements which reflected the fat thickness in both the upper and lower back.
BFT at L1 and L5 varied in their ability to explain oedema. BMI and BFT at L1 explained oedema to a similar extent and unlike BFT L5 are significant independent variables predicting oedema. These results are compatible with other studies which indicate that different fat deposits have different physiological functions and do not show identical correlation to BMI in explaining various disease processes. The fact that the mean thickness at L1 was approximately 28% less than that at L5 but L1 predicted oedema to a greater extent than L5 makes it more likely that the oedema may be a patho-physiological response. This is so because the finding suggests that, as with fat at other anatomical sites, fat different sites in the back may be associated with different pathologies. Our findings raise the possibility that BFT L5 may offer relative protection from microvascular dysfunction compared to BFT L1 which may be a predictor of micro-vascular dysfunction.
Shi et al. [1] concluded that being heavier and of female sex predisposed to the presence of oedema in the soft tissues of the lumbar region on MR. Our study however, indicates that sex per se is not a significant predictor of oedema but BMI, BFT and age are. The mean BMI of 30.3 kg/m2 of patients with oedema is indicative of obesity. Females are more likely to have oedema because of their significantly larger BMIs and BFTs. The oedema occurs in the adipose layers and the greater adipose content of females may in some way be related to their greater likelihood to show oedema.
Obesity may predispose to oedema because of the association with increased intra-abdominal pressure. Freeza et al. have reported that “For every 1 kg/mm2 increase in BMI, there was on average a 0.07 mm Hg increase in opening pressure” which in turn increases the pressure in the inferior vena cava [14]. The latter is likely to cause increase in the filtration of fluid into the interstitium because of increase in venous pressure. Obesity is also associated with expanded circulatory volume, which could result in increased intra-vascular pressure [15]. Additionally, obesity is associated with micro-vascular dysfunction. Tesauro et al. reported “obesity-related changes in vascular smooth muscle seem to disrupt the physiological facilitatory action of insulin on the responsiveness to vasodilator stimuli, whereas the adventitia and the perivascular fat appear to be a source of pro- inflammatory and vasoactive factors that may contribute to endothelial and smooth muscle cell dysfunction [16]. Other authors have reported on changes in the micro – vasculature in obesity [17]. MRI may be therefore be detecting oedema at an early stage before it becomes symptomatic or is able to be detected clinically in some patients. In other patients it may be detecting evidence of micro-vascular dysfunction.
A possible clinical utility of reporting on the presence of oedema is that it may prompt the clinician to evaluate the patient for other relevant diseases Thompson et al. have postulated that ‘Regardless of the aetiology…. small vessel disease is a systemic condition with major healthcare consequences, requiring a new paradigm in the way we practice medicine’ [18]. Greenstein et al. have reported on the association between cerebral microvascular damage in elderly depressed patients with structural and functional abnormalities of subcutaneous small arteries”. They found “profound abnormalities in both structure and function of small arteries, suggestive of deficiencies in local autoregulation” in biopsies of subcutaneous fat from the patients’ gluteal region [19].
This latter consideration should also inform research to determine whether the finding of oedema may be a predictor of imminent cardiac or renal decompensation or reflect a degree of vascular dysfunction. A limitation on this study is that cardiac, renal and hepatic disease, which could be causes of oedema, were excluded from the patients’ histories rather than by clinical examination and laboratory investigation. Another limitation is that we did not review the patients’ drug history. Some medications could impact the presence of oedema.
5. Conclusion
We analysed the extent to which obesity markers BMI and BFT at L1 and L5 predicted oedema in the subcutaneous fat of the lumbar region on Fat Sat MRI sequences in adults with no history of fluid overload or inflammatory process in the lumbar fat.
We found that BMI and BFT at L1 were significant independent variables predicting oedema in the lumbar subcutaneous fat on MRI and that they predicted oedema to a similar extent. BFT L5 was not a significant independent variable predicting oedema. BFT L1, with mean thickness approximately 28% less than that of BFT L5, predicted oedema to a greater extent than BFT L5 indicating that fat at different sites in the back may have different physiological effects. The similarity with which BMI and BFT L1 predict oedema suggests that the oedema may be, at least in part, a patho-physiological response to obesity.
Declarations
Author contribution statement
Wayne West: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data, Wrote the paper.
Keon P. West: Analyzed and interpreted the data.
Doreen Brady-West: Contributed reagents, materials, analysis tools or data, Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Shi H., Schweitzer M.E., Carrino J.A., Parker L. MR imaging of the lumbar spine: relation of posterior soft-tissue like-like signal and body weight. AJR Am. J. Roentgenol. 2003;180(1):81–86. doi: 10.2214/ajr.180.1.1800081. PMid:12490482. [DOI] [PubMed] [Google Scholar]
- 2.Genu A., Koch G., Colin D., Aho S., Pearson E., Ben Salem D. Factors influencing the occurrence of a T2-STIR hypersignal in the lumbosacral adipose tissue. Diagn. Interv. Imaging. 2014;95(March (3)):283–288. doi: 10.1016/j.diii.2013.10.005. Epub 2013 Nov 11. [DOI] [PubMed] [Google Scholar]
- 3.West W.M., West K.P. Edema in the lumbar subcutaneous fat, on routine MRI, of patients with no history of cardiac, renal or hepatic disease, is significantly associated with obesity and age. WIMJ Open. 2015 [Google Scholar]
- 4.Ujan J.A., Zan L.S., Ujan S.A., Adoligbe C., Wang H.B. Back fat thickness and meat tenderness are associated with a 526 T → a mutation in the exon 1 promoter region of the MyF-5 gene in Chinese Bos taurus. Genet. Mol. Res. 2011;10(December (4)):3070–3079. doi: 10.4238/2011.December.12.6. PMid:22194162. [DOI] [PubMed] [Google Scholar]
- 5.Lee K.T., Byun M.J., Kang K.S., Park E.W., Lee S.H., Cho S. Neuronal genes for subcutaneous fat thickness in human and pig are identified by local genomic sequencing and combined SNP association study. PLoS One. 2011;6(February (2)) doi: 10.1371/journal.pone.0016356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morooka T., Niiyama M., Uchida E., Uemura M., Miyoshi K., Saito M. Measurement of the back fat layer in beagles for estimation of obesity using two-dimensional ultrasonography. J. Small Anim. Pract. 2001;42(February (2)):56–59. doi: 10.1111/j.1748-5827.2001.tb01992.x. PMid:11263698. [DOI] [PubMed] [Google Scholar]
- 7.Wallner-Liebmann S.J., Kruschitz R., Hübler K., Hamlin M.J., Schnedl W.J., Moser M. A measure of obesity: BMI versus subcutaneous fat patterns in young athletes and nonathletes. Coll. Antropol. 2013;37(June (2)):351–357. PMid:23940974. [PubMed] [Google Scholar]
- 8.Romero-Corral A., Montori V.M., Somers V.K., Korinek J., Thomas R.J., Allison T.G. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368:666–678. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 9.Franzosi M.G. Should we continue to use BMI as a cardiovascular risk factor? Lancet. 2006;368:624–625. doi: 10.1016/S0140-6736(06)69222-2. [DOI] [PubMed] [Google Scholar]
- 10.Deurenberg P., Deurenberg-Yap M., Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat percent relationship. Obes. Rev. 2002;3:141–146. doi: 10.1046/j.1467-789x.2002.00065.x. PMid:12164465. [DOI] [PubMed] [Google Scholar]
- 11.Després J.P., Nadeau A., Tremblay A., Ferland M., Moorjani S., Lupien P.J. Role of deep abdominal fat in the association between regional adipose tissue distribution and glucose tolerance in obese women. Diabetes. 1989;38:304–309. doi: 10.2337/diab.38.3.304. PMid:2645187. [DOI] [PubMed] [Google Scholar]
- 12.Eastwood S.V., Tillin T., Dehbi H.M., Wright A., Forouhi N.G., Godsland I. Ethnic differences in associations between fat deposition and incident diabetes and underlying mechanisms: the SABRE study. Obesity (Silver Spring) 2015 doi: 10.1002/oby.20997. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross R., Fortier L., Hudson R. Separate associations between visceral and subcutaneous adipose tissue distribution, insulin and glucose levels in obese women. Diabetes Care. 1996;19:1404–1411. doi: 10.2337/diacare.19.12.1404. PMid:8941472. [DOI] [PubMed] [Google Scholar]
- 14.Frezza E.E., Shebani K.O., Robertson J., Wachtel M.S. Morbid obesity causes chronic increase of intraabdominal pressure. Dig. Dis. Sci. 2007;52(4):1038. doi: 10.1007/s10620-006-9203-4. PMid:17342401. [DOI] [PubMed] [Google Scholar]
- 15.Lavie C.J., Messerli F.H. Cardiovascular adaptation to obesity and hypertension. Chest. 1986;90(2):275–279. doi: 10.1378/chest.90.2.275. [DOI] [PubMed] [Google Scholar]
- 16.Tesauro M., Cardillo C. Obesity, blood vessels and metabolic syndrome. Acta Physiol. (Oxf.) 2011;203(1):279–286. doi: 10.1111/j.1748-1716.2011.02290.x. PMid:21439028. [DOI] [PubMed] [Google Scholar]
- 17.Valensi P., Smagghue O., Paries J., Velayoudon P., Lormeau B., Attali J.R. Impairment of skin vasoconstrictive response to sympathetic activation in obese patients: influence of rheological disorders. Metabolism. 2000;49:600–606. doi: 10.1016/s0026-0495(00)80034-7. [DOI] [PubMed] [Google Scholar]
- 18.Thompson C.S., Hakim A.M. Living beyond our physiological means: small vessel disease of the brain is an expression of a systemic failure in arteriolar function: a unifying hypothesis. Stroke. 2009;40(5):e322–e330. doi: 10.1161/STROKEAHA.108.542266. PMid:19228835. [DOI] [PubMed] [Google Scholar]
- 19.Greenstein A.S., Paranthaman R., Burns A. Cerebrovascular damage in late-life depression is associated with structural and functional abnormalities of subcutaneous small arteries. Hypertension. 2010;56(4):734–740. doi: 10.1161/HYPERTENSIONAHA.110.152801. PMid:20713917. [DOI] [PubMed] [Google Scholar]