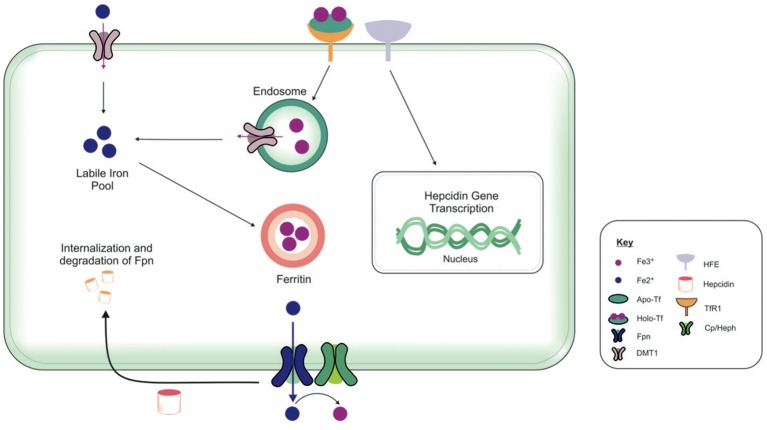
Figure 4.
Overview of cellular metabolism of iron in the brain. Transferrin (Tf) laden with iron (holo-Tf) binds to the Tf receptor-1 (TfR1), and iron enters the cell by receptor-mediated endocytosis and into endosomes (Moos and Morgan, 2004; Moos et al., 2007). Iron can be released from endosomes into the cytosolic labile iron pool, from here, iron can either be utilized in cellular processes or stored in ferritin (Ward et al., 2014). Additionally, ferrous iron (Fe2+) may enter cells via divalent metal transporter-1 (DMT1) into the cytosolic labile iron pool, but only a relatively small amount of ferrous iron is transported by this way. Excess Fe2+ may also be exported from the cell by ferroportin (Fpn), aided by membrane-bound ferroxidase ceruloplasmin (only present in astrocytes) (De Domenico et al., 2007). Ceruloplasmin is not known to regulate the Fpn-mediated export but stabilizes Fpn. Ferroxidases (hephaestin and ceruloplasmin) in the circulation or the cell surface oxidize Fe2+ to ferric (Fe3+) iron and facilitates binding to apo-Tf to form holo-Tf for vasculature transport. Hepcidin in the extracellular space inhibits iron export by binding to Fpn and mediates Fpn internalization and degradation (Ganz and Nemeth, 2012). The extracellular iron status modulates the cellular hepcidin levels by interactions between TfR1 and HFE TfR1which in turn regulates cellular hepcidin levels via unknown mechanisms. The key proteins involved in peripheral and central iron homeostasis are similar. However, the brain has its own unique regulatory mechanisms, as it is isolated by the cellular barriers–the blood brain and blood-CSF barriers. The exact way in which the different brain cell types interact with one another to maintain iron homeostasis remains to be elucidated. Also, the mechanism underlying the cross-talk between the brain and the periphery to regulate global iron homeostasis is not fully characterized.