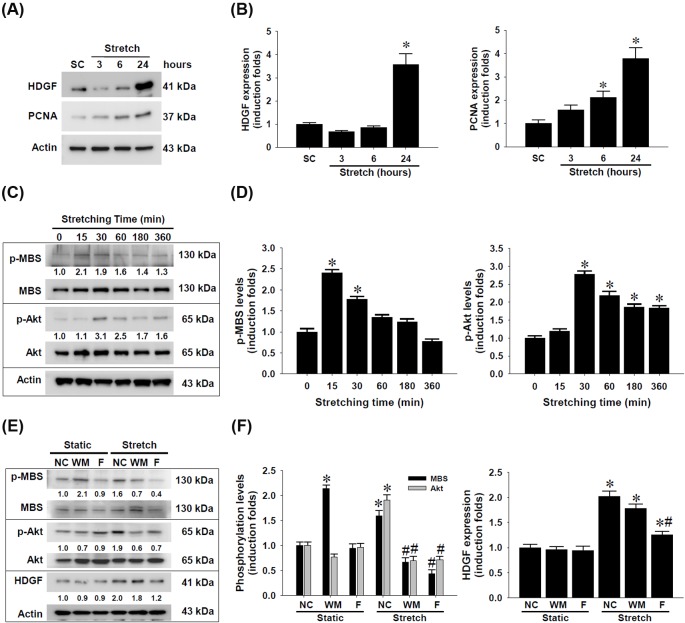
Figure 5. Up-regulation of HDGF expression in rat aortic smooth muscle cells (SMCs) by cyclic mechanical stretch and the signaling activity involved.
SMCs (8 × 105 cells per chamber) were seeded on fibronectin-coated silicone elastomer chambers and received uniaxial and cyclic 10% stretches at constant frequency (1 Hz). (A) Protein lysates of SMCs receiving cyclic mechanical stretch for indicated time and static control (SC) collected at 24 h were subjected to Western blotting detection for HDGF and proliferative cell nuclear antigen (PCNA) as a proliferative marker. (B) Densitometric analysis of HDGF and PCNA expression levels. (C) SMCs grown on fibronectin-coated elastomer chambers received mechanical stretch at the indicated time and the protein lysates were subjected to Western blot detection of MBS and Akt phosphorylation levels. (D) Densitometric analysis of MBS and Akt phosphorylation levels. (E) Crosstalk of RhoA/ROCK and PI3K/Akt signaling cascades and their involvement in cyclic mechanical stretch-induced HDGF up-regulation in SMCs. The cells were treated with wortmannin (WM) or fasudil (F) at 10 μM for 1 h and received uniaxial and cyclic 10% stretches at frequency (1 Hz) for 1 h. The protein lysates were subjected to Western blot detection of HDGF and phosphorylation levels of MBS and Akt proteins. (F) Densitometric analysis of MBS and Akt phosphorylation as well as HDGF expression levels. The representative blot images obtained from three independent experiments are shown with induction folds compared with time zero or static negative control (NC). Density data are shown in mean ± SEM; *P<0.05 compared with SC or time zero control; #P<0.05 compared with corresponding stretched NC.