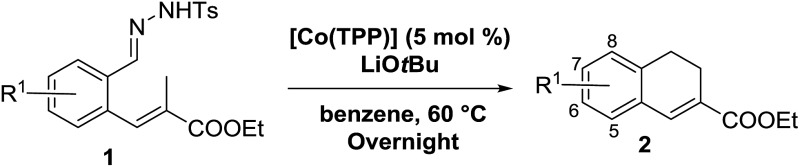
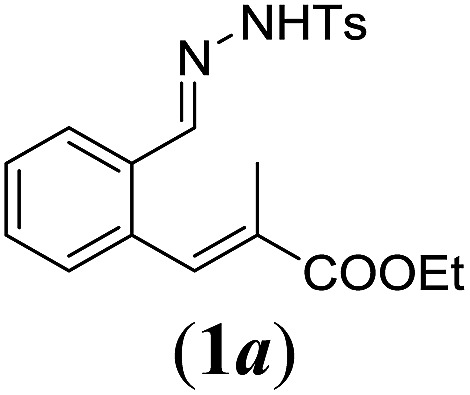
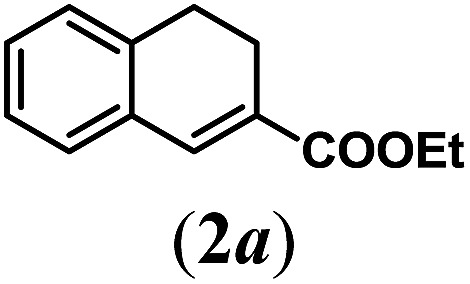
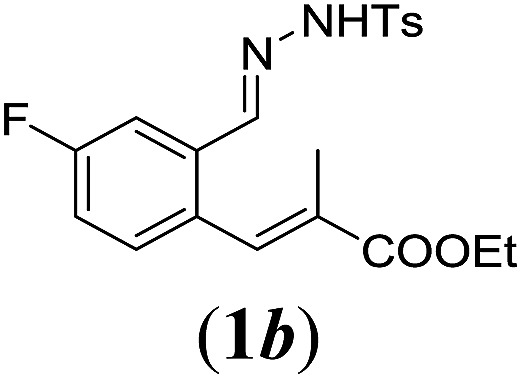
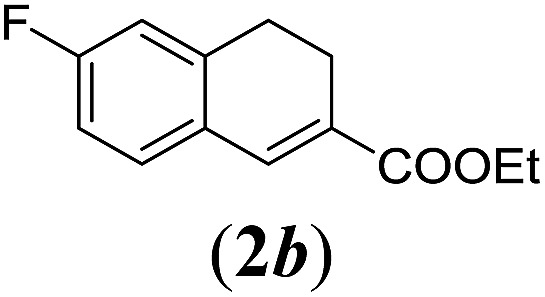
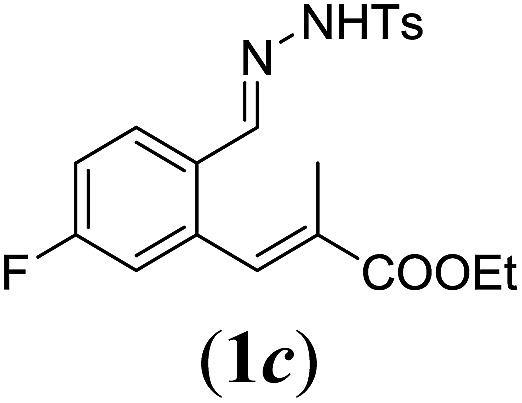
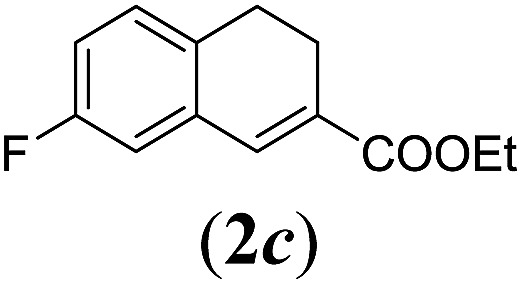
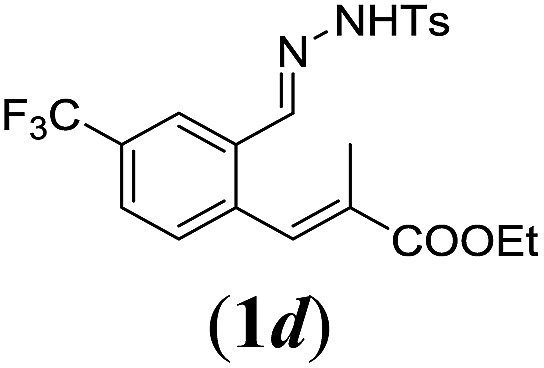
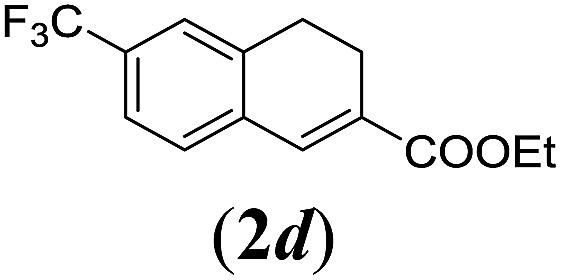
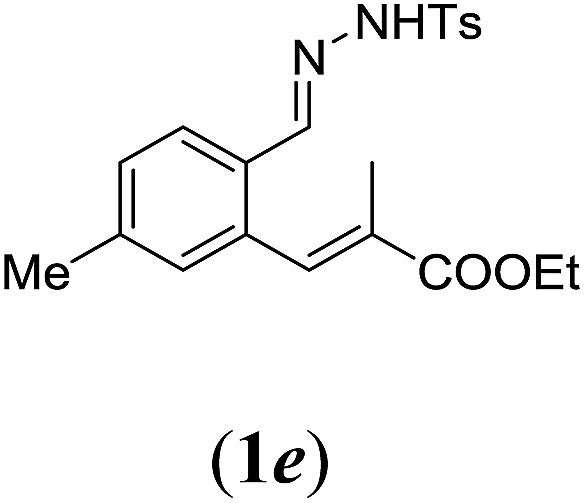
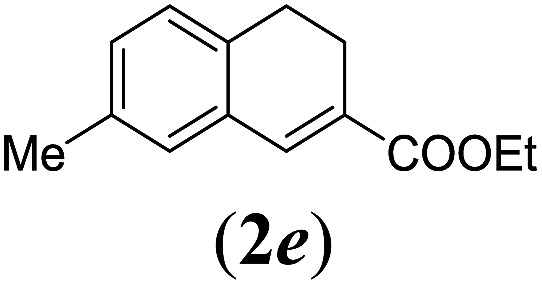
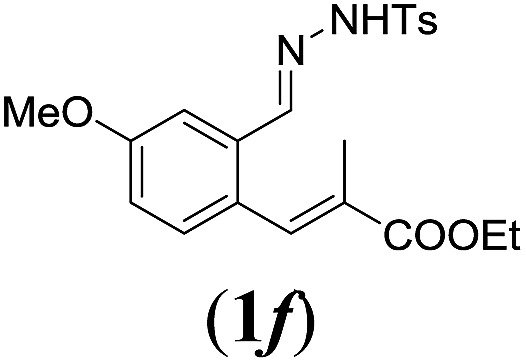
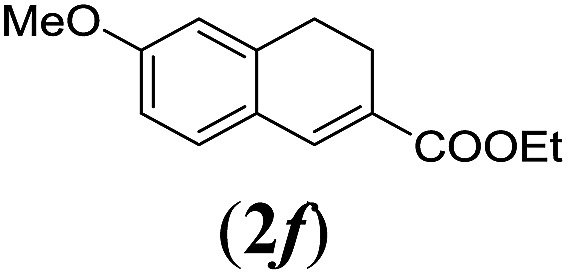
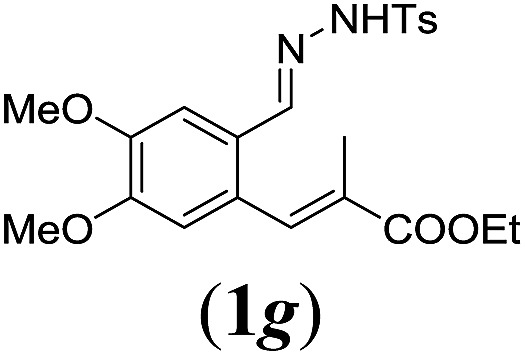
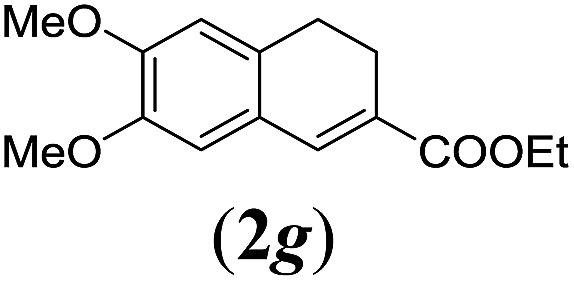
Table 2. Substrates scope varying functional groups R1 at the aromatic ring a .
aReaction conditions: N-tosylhydrazone (1a–g) (0.1 mmol, 1.0 equiv.), LiOtBu (0.12 mmol, 1.2 equiv.), [Co(TPP)] (5 mol%), benzene (2 mL), 60 °C, overnight.
bIsolated yields after column chromatography (average of two separate experiments).
cIsolated yield of experiment performed with 1.0 mmol substrate.
dYields of these three entries based on the major E-isomer, using an E/Z-mixture containing 6–12% of the non-productive Z-isomer.