Figure 3.
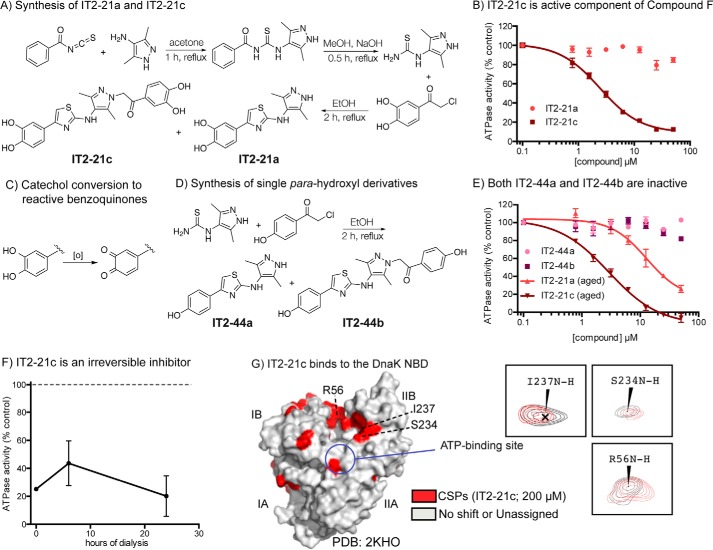
Side product of compound F synthesis is an irreversible inhibitor of Hsp70 systems. A, synthesis of IT2-21a and IT2-21c by Hantzsch thiazole condensation. Compounds IT2-21c and IT2-21a were separated by HPLC. B, IT2-21c, not IT2-21a, is an inhibitor of DnaK/DnaJ/GrpE ATPase activity. Results are the average of triplicates, and error bars represent S.E. C, known oxidation of catechol rings to yield reactive benzoquinones. D, synthesis of IT2-44a and IT2-44b. Compounds were separated by HPLC. E, neither of the para-phenolic analogs were active in the ATPase assay using DnaK/DnaJ/GrpE. Results are the average of triplicates, and error bars represent S.E. “Aging” DMSO stocks of IT2-21a and IT2-21c by overnight incubation at room temperature improved their activity, consistent with an oxidative mechanism. F, IT2-21c (100 μm) was incubated with DnaK (2.5 μm) and then subjected to dialysis. Samples were removed from dialysis after 6 and 24 h, and the ATPase assay was performed with added DnaJ. Even 24 h of dialysis was not able to reverse compound activity. G, a potential binding site of IT2-21c was revealed by HSQC experiments with 15N-labeled DnaK NBD. CSPs (>0.01 ppm in 1H and/or >0.1 ppm in 15N) in the presence of IT2-21c were localized to the IB and IIB subdomains (red). See under “Materials and methods” for details. Peaks shifting for select residues are highlighted with NBD + 2% DMSO in gray and NBD + 200 μm IT2-21c in red.