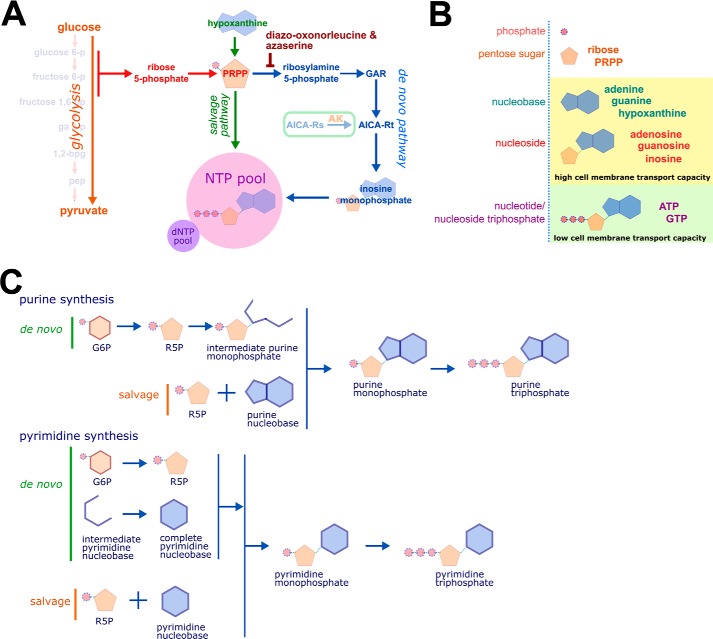
Figure 4.
Nucleotide synthesis, structure, and nomenclature. A, ribose 5-phosphate (R5P) is produced by the metabolism of glucose 6-phosphate (G6P) through the pentose phosphate pathway (PPP). Phosphoribosyl pyrophosphate (PRPP) is essential for both salvage purine synthesis and de novo purine synthesis. The inhibitors DON and azaserine inhibit enzymes in the de novo purine synthesis pathway proximal to the intermediate AICA-Rt. The nucleoside AICA-Rs is readily transported across the cell membrane and is phosphorylated by adenosine kinase to the de novo synthesis pathway intermediate AICA-Rt. B, nucleobases and nucleosides are readily transportable across the cell membrane. Nucleotides (phosphonucleosides) have low cell membrane permeability. C, de novo and salvage purine synthesis both depend on PRPP. The carbon 5′-phosphate moiety of PRPP, originally added to glucose by hexokinase, defines purine synthesis pathway intermediates as nucleotides. In de novo synthesis, the purine ring is built directly onto PRPP. In contrast, the pyrimidine nucleobase is synthesized independently of PRPP, which is added to the complete nucleobase to form a pyrimidine nucleotide. GAR, glycinamide ribonucleotide; NTP, nucleotide triphosphate; AK, adenosine kinase; arrows represent multistep metabolic pathways.