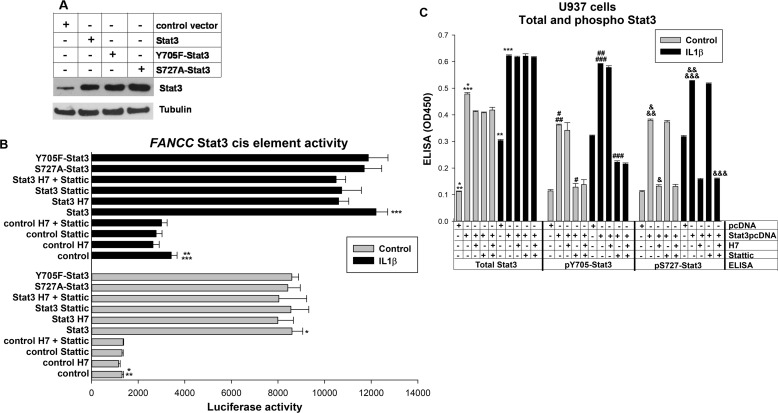
Figure 4.
Stat3 protein abundance contributes to FANCC promoter activation. A, Stat3, Y705F-Stat3, and S727A-Stat3 are equivalently overexpressed in U937 cells. U937 cells were transfected with vectors to overexpress various Stat proteins. Protein expression was determined by Western blots serially probed with antibodies for Stat3, Tyr(P)-705–Stat3, Ser(P)-727–Stat3, or tubulin (as a loading control). A representative blot is shown. B, increased abundance of Stat3 protein increases the activity of the FANCC promoter Stat3-binding cis element. U937 cells were transfected with a minimal promoter/luciferase reporter construct with −438 to −474 bp of FANCC 5′ flank (or the minimal promoter/luciferase reporter control vector). Cells were co-transfected with vectors to overexpress Stat3, S727A-Stat3, or Y705F-Stat3 (versus control vector). Some transfectants were treated with a serine kinase inhibitor (H7), a tyrosine kinase inhibitor (Stattic), or both, and some were differentiated with IL1β prior to analysis. Statistically significant differences are indicated by *, **, or *** (p < 0.01, n = 6 for all comparisons). C, H7 or Stattic alter the Stat3 phosphorylation state but not protein abundance. U937 cells were transfected and assayed under the conditions described for reporter gene assays. Total, Tyr(P)-705–Stat3, or Ser(P)-727–Stat3 were analyzed by ELISA. Statistically significant differences are indicated by *, **, ***, #, ##, ###, &, &&, or &&& (p < 0.01, n = 6 for all comparisons).