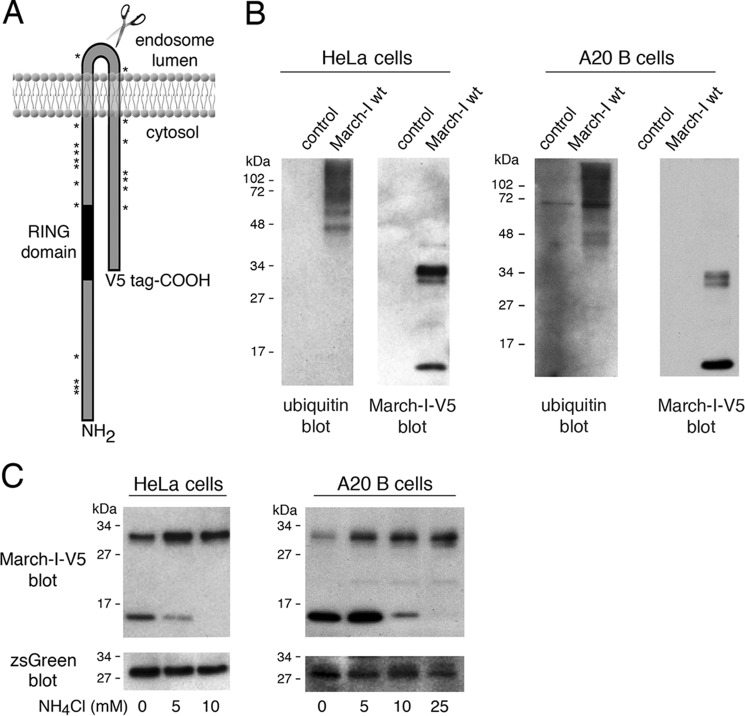
Figure 1.
March-I is ubiquitinated and undergoes proteolytic degradation. A, schematic of the structure of March-I, showing the location of the C-terminal V5 epitope tag, the catalytic RING domain, and the location of each of the 19 Lys residues in the full-length 272 amino acid human March-I (variant 2) protein. B, HeLa cells (left panel) or A20 B cells (right panel) were transfected with an expression vector encoding V5 epitope–tagged March-I containing an IRES and a zsGreen1 protein reporter or the same IRES/zsGreen1 protein reporter alone as a control. After 18 h, cells were harvested and lysed, and each sample was incubated with anti-V5-agarose beads. The samples were analyzed by immunoblotting using anti-ubiquitin or anti-V5 antibodies. C, HeLa cells (left panel) or A20 B cells (right panel) were transfected with an expression vector encoding V5 epitope–tagged March-I containing an IRES and a zsGreen1 protein reporter. After 4 h, the cells were washed and incubated in complete medium containing the indicated concentration of NH4Cl for an additional 16 h before harvesting. Cell lysates were prepared, and aliquots of each lysate were analyzed for expression of V5-March-I as well as the zsGreen1 reporter. Representative gels from more than three independent experiments are shown.