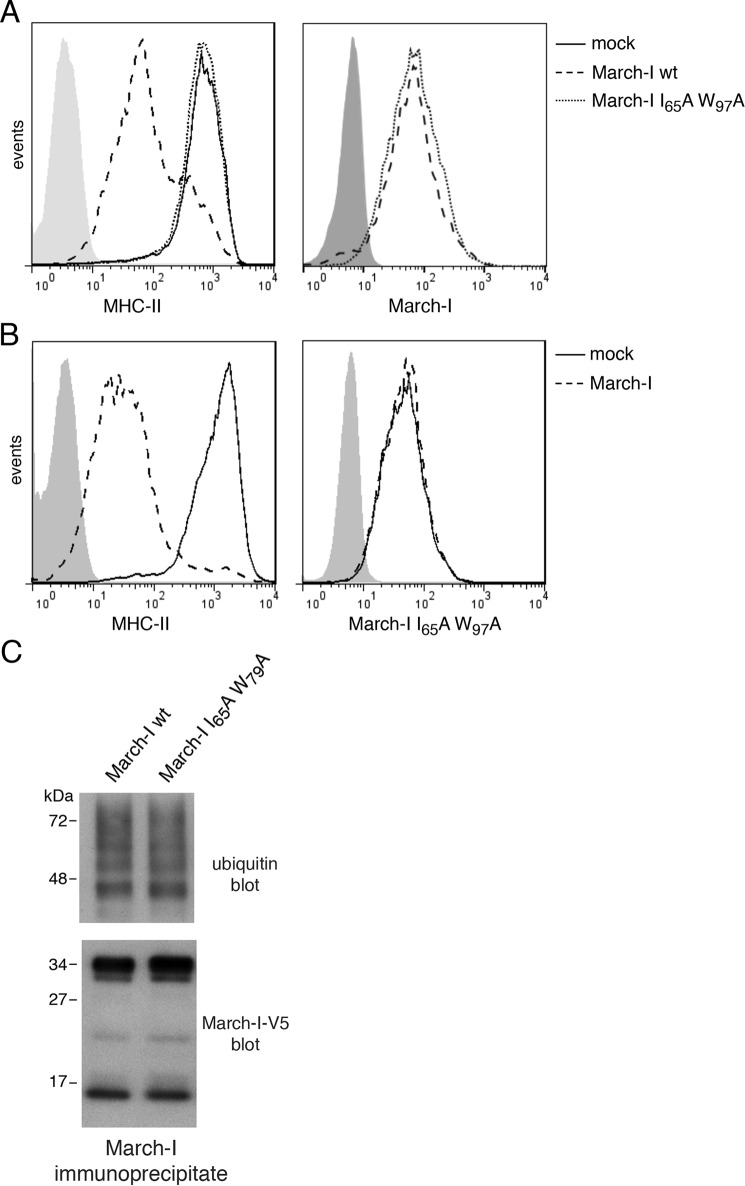
Figure 2.
March-I E3 ligase activity is not required for its ubiquitination. HeLa-CIITA cells were transfected with an expression vector encoding V5-tagged wildtype March-I/IRES/zsGreen1 protein, a V5-tagged I65A/W97A RING domain mutant in the IRES/zsGreen1 vector, or the IRES/zsGreen1 protein reporter alone as a control. After 18 h, cells were harvested and analyzed. A, expression of MHC-II on the surface of live cells (left panel) or V5-March-I present in fixed/permeabilized cells (right panel) was analyzed by FACS. B, HeLa-CIITA cells were co-transfected with vectors expressing a V5-tagged March-I I65A/W97A RING domain mutant together with FLAG-tagged March-I or an empty vector control. After 18 h, cells were harvested, and the expression of MHC-II on live cells (left panel) or V5-tagged I65A/W97A RING domain mutant March-I present in fixed/permeabilized cells (right panel) was analyzed by FACS. Representative FACS profiles from three independent experiments are shown. C, lysates of cells transfected with the V5 epitope–tagged wildtype or I65A/W97A RING domain March-I mutant were incubated with anti-V5-agarose beads to immunoprecipitate March-I, and the extent of March-I ubiquitination in each sample was determined by anti-ubiquitin and anti-V5 immunoblotting. A representative blot of three independent experiments is shown.