Figure 3.
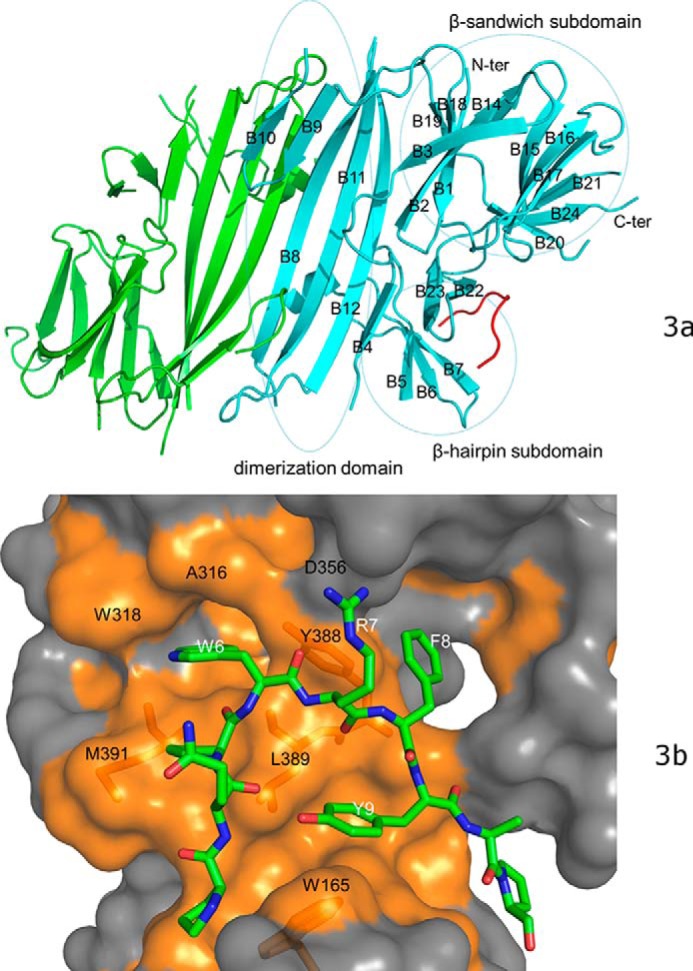
The crystal structure of bovine PERK luminal domain complexed with the peptide substrate P16 determined to 2.8-Å resolution. a, the PERK luminal domain homodimer structure. One of the monomers is ligand-free (in green) and the other is complexed with the peptide substrate P16 (in cyan). The peptide substrate P16 is shown in red. The N terminus and C terminus of the protein are labeled. The 24 β-strands in the complex monomer are labeled as B1–B24. The dimerization domain, β-sandwich subdomain, and β-hairpin subdomain are circled and labeled. b, the surface drawing for the PERK luminal domain complexed with the peptide substrate P16. The PERK luminal domain is shown in surface drawing and the peptide substrate P16 is shown in stick mode. The hydrophobic patches within the peptide-binding groove of the PERK luminal domain is shown in gold. The conserved residues from the peptide-binding grooves that are involved in binding the peptide substrate are labeled in black. Residues Trp-165, Tyr-388, Leu-389, and Met-391 of the PERK luminal domain are shown under the semi-transparent surface. The residues from the peptide substrate P16 that make major contacts with the PERK luminal domain are labeled in white.
