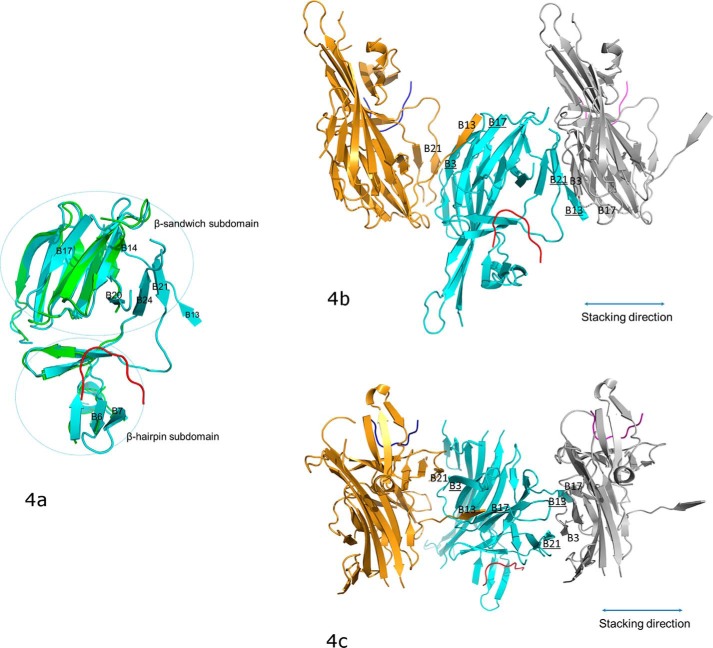
Figure 4.
The conformational changes of PERK luminal domain after peptide substrate binding that mediate the PERK oligomerization. a, the superimposition of the peptide-binding domains for the ligand-free monomer (in green) and the peptide-binding monomer (in cyan). The peptide substrate is in red. The β-strands where major conformational changes occur after the peptide binding are labeled. The orientation of this panel is similar to that in Fig. 3b. b, the stacking format revealed by the crystal packing for the PERK luminal domains complexed with peptide substrates. The middle PERK luminal domain complex monomer (cyan) is stacked by two neighboring complex monomers that are translated by 1 unit cell along the a axis. The stacking interactions involve two β-strand formations on either side of the middle monomer complex. On the left side, B3 and B17 (underlined) from the middle monomer form β-strands with B21 and B13 from the left monomer complex (in gold). On the right side, B13 and B21 (underlined) from the middle monomer complex form β-strands with B17 and B3 from the right monomer complex (in silver). This stacking format will allow PERK molecules to oligomerize along the stacking direction (shown in double-ended line) to both ends by any number. The orientation for the middle monomer in this panel is similar to that in panel a and Fig. 3b. c, this panel is generate by rotating b by ∼90° along the horizontal axis.