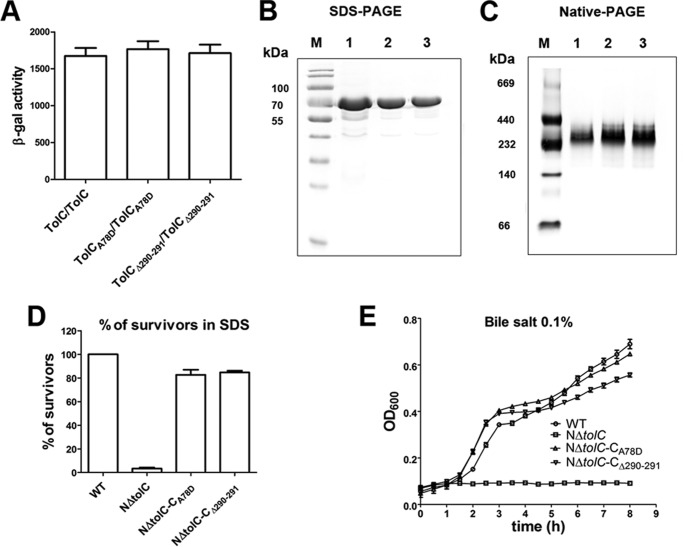
Figure 7.
Analysis of the mass, oligomeric states, and the physiological function of TolCA78D or TolCΔ290–291. A, BACTH assays of the trimerization of each TolC mutant protein with A78D and Gly-290–Glu-291 deletion, respectively. The resulting recombinant plasmid pair pT25-TolCA78D/pT18C-TolCA78D or pT25-TolCΔ290–291/pT18C-TolCΔ290–291 was co-transformed into BTH101, and the β-galactosidase activity was measured. TolC/TolC was used as the positive control. B, TolC-GST, TolCA78D-GST, and TolCΔ290–291-GST protein were separated by SDS-PAGE. A 74-kDa band representing the single subunit of TolC is shown. C, protein samples were separated by native-PAGE (4–15%, Solarbio). A 240-kDa band showing the trimer of TolC is shown. Lane M indicates mass. D, analysis of the SDS sensitivity of strains expressing TolCA78D or TolCΔ290–291 proteins. The cfu of each sample was normalized by cfu counting without SDS. E, growth kinetics of wildtype strain N16961 (WT), NΔtolC, NΔtolC-CA78D, and NΔtolC-CΔ290–291 in the presence of 0.1% bile salt.