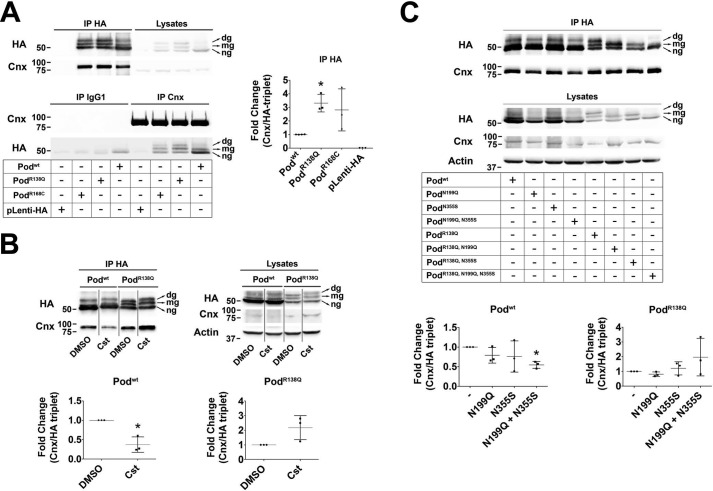
Figure 5.
PodocinR138Q has an enhanced interaction with calnexin. A–C, co-immunoprecipitation analyses of podocin and calnexin in HEK293T cells. A, co-immunoprecipitation of HA-tagged Podwt, PodR138Q, PodR168C, or an empty HA lentiviral vector (pLenti-HA) with Cnx. B, cells were treated with the glucosidase I and II inhibitor castanospermine (Cst; 500 μm, 16 h) before performing HA-immunoprecipitation to study the lectin-dependent interaction of Cnx with Podwt and PodR138Q. C, co-immunoprecipitation of Cnx with podocin in cells overexpressing HA-tagged Podwt and PodR138Q with or without mutated N-glycosylation sites Asn199 and Asn355. A–C, monoclonal anti-HA and anti-Cnx AF18 were used to identify podocin and Cnx, respectively. Graphs represent the densitometry quantification of Cnx when podocin is immunoprecipitated (IP HA). Data are normalized to total immunoprecipitated podocin (HA triplet in IP HA) and represent at least three independent experiments. *, p < 0.05.