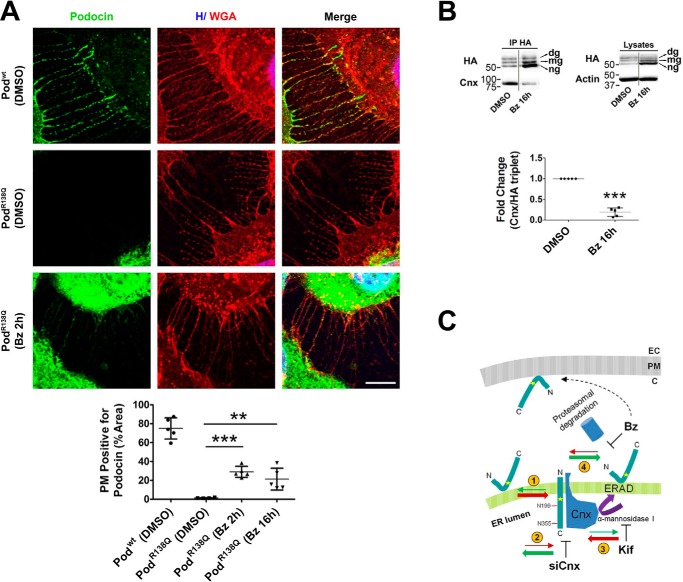
Figure 7.
Bortezomib partially re-addresses podocinR138Q to the plasma membrane. A, immunofluorescence analysis showing PodR138Q (green) in the presence of filopodia, through colocalization with the PM marker WGA (red), after a 2-h treatment with Bz (0.1 μm) or vehicle (DMSO). Podwt co-localization with WGA is included as a positive control. Hoechst nuclei labeling (H) is also shown (blue). Scale bar = 10 μm. Image analysis of the WGA co-localization with podocin only at filopodia was obtained through the quantification of regions of interest (ROIs) that carefully delimit cell perimeters. Graph corresponds to the quantification of one representative experiment. One-way analysis of variance followed by Dunnett's post-test was used as statistical analysis. **, p < 0.01 and ***, p < 0.001. B, co-immunoprecipitation (IP HA) of HA-podocin and Cnx after Bz addition (16 h at 0.1 μm). The graph represents the densitometry quantification of Cnx when podocin is immunoprecipitated. Data are normalized to total immunoprecipitated podocin (HA triplet) and represent to at least three independent experiments. ***, p < 0.001. C, schematic summarizing the influence of different treatments on PodR138Q topology and subcellular localization. Red and green arrows indicate a dynamic change to the transmembrane “wrong” or hairpin “right” topology, respectively. Numbers circled in yellow: (1) there is an imbalance of hairpin PodR138Q toward a transmembrane topology, (2) decreasing PodR138Q interaction with Cnx through siCnx transfection favors the hairpin topology, (3) stabilization of PodR138Q interaction with Cnx, through inhibition of Cnx cycle exit with Kif, enhances podocin transmembrane topology, and (4) inhibition of PodR138Q proteasomal degradation with Bz increases the proportion of hairpin PodR138Q and is the only treatment that allows partial relocalization to the PM. EC, extracellular matrix; PM, plasma membrane; C, cytosol.