Abstract
As a major cancer type in females, cervical cancer has been explored in depth by researchers. HeLa is a cervical cancer cell line. Isorhamnetin is an O-methylated flavonol that is primarily extracted from sea buckthorn. In the present study, the anti-proliferative effect of isorhamnetin on HeLa cells was evaluated using a Trypan blue dye exclusion assay. Isorhamnetin inhibited the cell proliferation in a time- and dose-dependent manner. Flow cytometric analysis of the cell cycle distribution revealed that isorhamnetin inhibited the cell cycle progression of HeLa by causing G2/M phase arrest and decreasing the proportion of cells in G1 phase. In addition, western blot analysis was performed to evaluate the presence of certain cell cycle-associated proteins. It was demonstrated that isorhamnetin inhibited the protein expression of cyclin B1, cell division cycle 25C (Cdc25C) and Cdc2, but enhanced checkpoint kinase 2 (Chk2), Cdc25C and Cdc2 phosphorylation. In addition, tubulin depolymerization participated in the isorhamnetin-induced cell cycle arrest in G2/M phase. In conclusion, the present results indicated that the anti-proliferative action of isorhamnetin is associated with arrest of the cell cycle in G2/M phase, which is a consequence of activation of the ataxia telangiectasia mutated Chk2 pathway and disruption of microtubule function.
Keywords: isorhamnetin, cervical cancer, anti-proliferation, cell cycle arrest, ataxia telangiectasia mutated checkpoint kinase 2, microtubule
Introduction
Cervical cancer is the third most common type of cancer among women worldwide, and the majority of cases are associated with infection caused by human papillomavirus (HPV) (1). Radiotherapy, surgery and chemotherapy are the current therapeutic options for the treatment of cervical cancer. However, these treatments are limited by high cost, high systemic toxicity, severity of side effects and drug resistance (2). The mortality rate for cervical cancer is relatively low in developed countries due to cancer screening and the use of the HPV vaccine. However, it is the major cause of cancer-associated mortality in women in developing countries (3). Thus, more effective and convenient anti-neoplastic treatments are required for cervical cancer.
Numerous types of naturally derived phytochemicals have been demonstrated to have anti-cancer effects on cervical cancer cell lines (4–6). In a previous study, curcumin restored p53, induced DNA damage and inhibited cell proliferation in HeLa cells (7). Ellagic acid also exhibited anti-tumor effects, since it arrested CaSki cells in G0/G1 phase and induced apoptosis (8).
Isorhamnetin, an O-methylated flavonol, is mainly extracted from sea buckthorn (Hippophae rhamnoides L.) (9). Previous studies revealed that isorhamnetin exerts multiple pharmacological functions, including anti-inflammatory, antioxidant and anticancer activities (10–12). Isorhamnetin has been reported to downregulate several inflammatory proteins, including cyclooxygenase-2, prostaglandin E2, tumor necrosis factor-α and nuclear factor κB (NF-κB) (11). Furthermore, isorhamnetin induced the expression of NF-E2-related factor 2-dependent antioxidant genes, resulting in reduced oxidative stress (13). In addition, isorhamnetin has been demonstrated to upregulate p53, activate the expression of the apoptotic factors B-cell lymphoma 2-associated X protein and caspase-2, and induce apoptosis in lung cancer cells (14). Recently, it has been reported that several isorhamnetin glycoside derivatives exhibit moderate antitumor activity in cervical cancer (15,16). However, to the best of our knowledge, the mechanism the antitumor effect of isorhamnetin on cervical cancer cell lines has remained elusive. Therefore, the present study investigated whether isorhamnetin exerts anti-proliferative effects on the human cervical cancer cell line HeLa.
Materials and methods
Isorhamnetin preparation
Isorhamnetin was from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). It was first dissolved in dimethyl sulfoxide (DMSO) to generate a stock solution. For cell treatments, the stock solution was further diluted in culture medium as required. The final concentration of DMSO in the culture medium was <0.4% (v/v).
Chemicals
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), propidium iodide and trypsin-EDTA were from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Bovine serum albumin (BSA), DMSO and Trypan blue were from Sigma-Aldrich (Merck KGaA). Penicillin and streptomycin were obtained from M&C Gene Technology (Beijing, China).
Antibodies
The primary antibodies to checkpoint kinase (Chk) 1 (cat. no. 2360), Chk2 (cat. no. 3440) and call division cycle (Cdc) 2 (cat. no. 9116), Cdc25C (cat. no. 4688), phosphorylated (p)-Cdc2 (Tyr15; cat. no. 4539), p-Chk1 (Ser345; cat. no. 2348), p-Chk2 (Thr68; cat. no. 2197) and p-Cdc25C (Ser216; cat. no. 4901), β-actin (cat. no. 4970) (all dilution 1:1,000) and cyclin B1 (cat. no. 4135; dilution 1:2,000) were obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA). Primary antibodies for α-tubulin (cat. no. ab7750) and β-tubulin (cat. no. ab70187) were obtained from Abcam (Cambridge, MA, USA) and used at dilution 1:500. The secondary antibodies for goat anti-rabbit (cat. no. A0208) and goat anti-mouse (cat. no. A0216) were purchased from Beyotime Institute of Biotechnology (Haimen, China) and used at dilution 1:1,000. For western blots, the antibodies were diluted in 0.5% blocking buffer (add 5 g BSA, 1.22 g Tris and 8.78 g NaCl to 1 l distilled water and adjust pH to 7.5).
Cell culture
HeLa cells were obtained from Bioleaf Company (Shanghai, China) and cultured in DMEM supplemented with 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin. The cells were maintained in a humidified atmosphere of 5% CO2 at 37°C and were passaged every 2–3 days.
Cell proliferation assay
The anti-proliferative activity of isorhamnetin was measured using a Trypan blue dye exclusion assay (17). HeLa cells were seeded in a 96-well plate at 5,000 cells/well. After 12 h, various concentrations of isorhamnetin (0, 1, 10, 100 or 1,000 µmol/l) were applied to the cells for 24, 48 or 72 h. The cells were then trypsinized and re-suspended in PBS. Trypan blue dye solution (0.4%) was added to the cell suspension. After 2 min, the number of colored (dead) cells and unstained (viable) cells per mm2 was counted under a phase contrast microscope. Results were expressed as the mean ± standard deviation of six independent experiments. The IC50 of isorhamnetin was determined using SPSS Statistics version 19.0 (IBM Corp., Armonk, NY, USA).
Flow cytometric analysis
The cell cycle distribution was assessed by flow cytometry (18). HeLa cells were first cultured at a density of 1×106 cells/100 mm for 24 h and then the medium was replaced with fresh medium containing various concentrations of isorhamnetin. After incubation for 24 or 72 h, cells were stained with propidium iodide and analyzed using a flow cytometer (BD Accuri™ C6; BD Biosciences, Franklin Lakes, NJ, USA). Data analysis was performed using BD CellQuest™ cell cycle analysis software version 5.1 (BD Biosciences). The experiments were performed in triplicate.
Western blot analysis
HeLa cells were treated with different concentrations of isorhamnetin for 24 h. Subsequently, the cells were washed with PBS. For the extraction of total protein, cells were collected by scraping and incubated for 1 h in sample buffer [150 mmol/l sodium chloride, 20 mmol/l Tris (pH 7.5), 1 mmol/l EDTA, 2.5 mmol/l sodium pyrophosphate, 1 mmol/l β-glycerophosphate, 1% Triton X-100, 1 mmol/l sodium orthovanadate, 1 mmol/l phenyl methane sulfonyl fluoride, 0.5% sodium deoxycholate, 1% protease inhibitor and 20 mmol/l sodium fluoride], and then centrifuged at 12,000 × g for 30 min at 4°C. The total protein concentration in the clear supernatant was evaluated using the Bradford method. Aliquots containing 30 µg protein were subjected to 15% SDS-PAGE. The proteins were then electrophoretically transferred to a nitrocellulose membrane (cat. no. HATF00010; EMD Millipore, Billerica, MA, USA). Membranes were blocked for 1 h at room temperature with a 3% blocking buffer (add 30 g BSA in Tris-buffered saline (TBS) buffer to final volume 1 l). The membranes were rinsed with TBS buffer (add 1.22 g Tris and 8.78 g NaCl to 1 l distilled water and adjust pH to 7.5 with HCl) three times, for 5 min each time. The primary antibodies were added in 10 ml 0.5% blocking buffer and incubated for 2 h at room temperature. The membranes were washed twice for 10 min each time with Tween-20 TBS buffer (add 0.5 ml 0.05% Tween-20 to 1 l TBS buffer). The secondary antibodies were added in a 5 ml 0.5% blocking buffer and incubated for 1 h at room temperature. The membranes were washed twice for 10 min with Tween-20 TBS buffer. Specific signals were detected by enhanced chemiluminescence (Amersham International; GE Healthcare, Little Chalfont, UK). The densitometry was performed using ImageJ software (National Institute of Health, Bethesda, Maryland, USA) and the density of each band was normalized against β-actin. All experiments were performed in triplicate.
Statistical analysis
Differences between the control and treatment groups were analyzed by analysis of variance, followed by Duncan's multiple range test. SPSS Statistics version 19.0 was used for statistical analysis. P<0.05 was considered to indicate a statistically significant difference. Values are expressed as the mean ± standard deviation of at least three independent experiments.
Results
Isorhamnetin inhibits HeLa cell proliferation
The cell proliferation assay demonstrated that isorhamnetin inhibited the proliferation of HeLa cells at concentrations of >10 µmol/l in a dose-dependent manner (Fig. 1). The IC50 values of isorhamnetin in HeLa cells were 100.03 µmol/l at 24 h, 304.15 µmol/l at 48 h and 54.79 µmol/l at 72 h.
Figure 1.
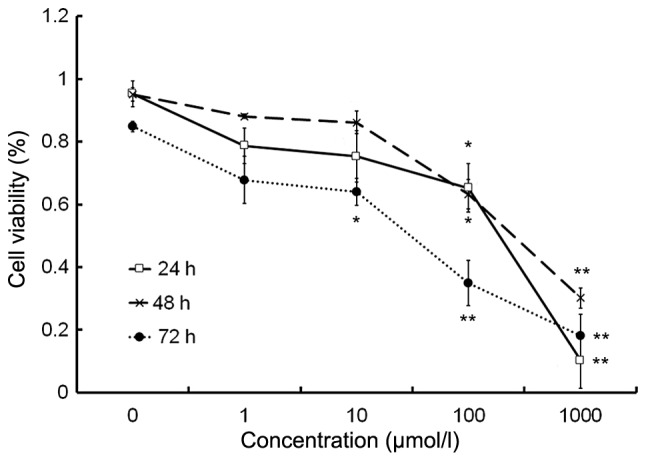
Isorhamnetin inhibits the proliferation of HeLa cells. Cells were exposed to different doses of isorhamnetin (1–1,000 µmol/l) for 24, 48 and 72 h. *P<0.05 and **P<0.01 vs. the control group. Values are expressed as the mean ± standard deviation of six independent experiments.
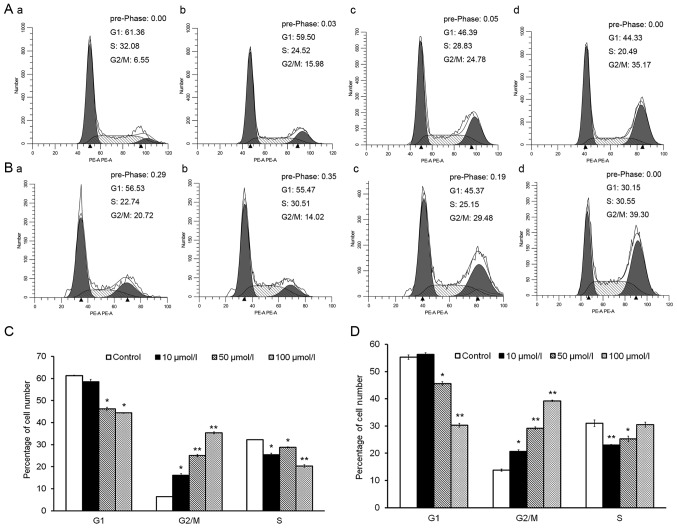
Isorhamnetin induces cell cycle arrest in G2/M phase
Cell cycle distribution analysis revealed that treatment with isorhamnetin for 24 and 72 h significantly increased the proportion of cells in G2/M phase (Fig. 2A and B). Upon treatment with 10, 50 or 100 µmol/l isorhamnetin for 24 h, the percentage of cells in G2/M phase rose 2.5-, 3.8- and 5.5-fold, respectively, compared with that in the control group (Fig. 2C). After treatment for 72 h, the same concentrations of isorhamnetin increased the percentage of cells in G2/M phase by 1.5-, 2.1- and 2.8-fold, respectively, compared with those in the control group (Fig. 2D). By contrast, treatment with various concentrations of isorhamnetin for 24 and 72 h decreased the percentage of HeLa cells in G1 phase in a dose-dependent manner (Fig. 2C and D). These results indicated that isorhamnetin causes cell cycle arrest at G2/M phase, accompanied by a decrease in the percentage of cells in G1 phase, in a dose-dependent manner in HeLa cells.
Figure 2.
Isorhamnetin arrests HeLa cells at G2/M phase. Flow cytometry was performed to assess the cell cycle distribution of HeLa cells treated with (A-a) 0, (A-b) 10, (A-c) 50 or (A-d) 100 μmol/l isorhamnetin for 24 h, or (B-a) 0, (B-b) 10, (B-c) 50 or (B-d) 100 μmol/l isorhamnetin for 72 h. The percentage of cells in G1, G2/M or S phase was assessed after treatment with various concentrations of isorhamnetin for (C) 24 and (D) 72 h. *P<0.05 and **P<0.01 vs. the control group. Values are expressed as the mean ± standard deviation of triplicate results.
Isorhamnetin alters the levels and activity of G2/M phase-regulatory proteins
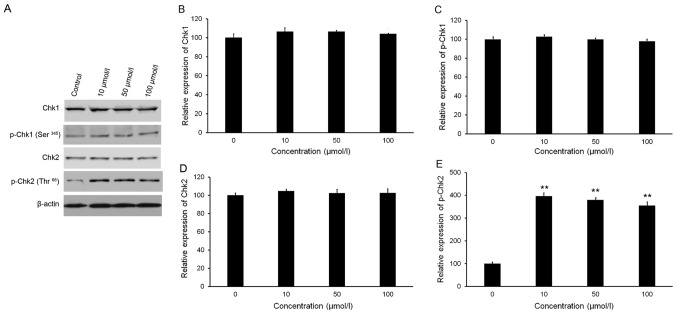
Isorhamnetin affects Chk2 phosphorylation
As presented in Fig. 3A-C, western blot analysis revealed no significant variations in the phosphorylation and total protein levels of Chk1 following treatment with isorhamnetin. Treatment with isorhamnetin markedly increased the phosphorylation of Chk2 (Thr68), but with no significant alteration in Chk2 total protein levels (Fig. 3A, D and E). Quantitative analysis indicated that the level of p-Chk2 in HeLa cells treated with 10, 50 and 100 µmol/l isorhamnetin was increased by 4.0-, 3.8- and 3.5-fold, respectively, compared with that in the control group (Fig. 3E).
Figure 3.
Effect of isorhamnetin on the levels of Chk1, p-Chk1 (Ser345), Chk2 and p-Chk2 (Thr68). The cells were treated with isorhamnetin (10, 50 or 100 µmol/l) for 24 h. (A) Protein levels were evaluated by western blot analysis. (B-E) Quantification of western blot results for (B) Chk1, (C) p-Chk1 (Ser345), (D) Chk2 and (E) p-Chk2 (Thr68). Protein levels were normalized to β-actin. The relative level of protein in the control group was set at 100%. **P<0.01 vs. the control group. Values are expressed as the mean ± standard deviation of triplicate results. p-Chk, phosphorylated checkpoint kinase.
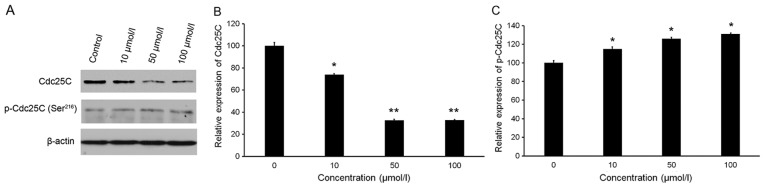
Isorhamnetin affects the protein levels of Cdc25C and p-Cdc25C
As presented in Fig. 4A and B, isorhamnetin slightly increased the protein levels of p-Cdc25C (Ser216). Conversely, isorhamnetin treatment significantly inhibited the protein expression of Cdc25C (Fig. 4A and C). Treatment with 10, 50 and 100 µmol/l isorhamnetin caused 26.15, 67.57 and 67.25% inhibition of Cdc25C levels, respectively, compared with the those in the control group (Fig. 4C).
Figure 4.
Effect of isorhamnetin on the levels of Cdc25C and p-Cdc25C (Ser216). (A) Protein levels were evaluated by western blot analysis. (B and C) Quantification of western blot results for (B) Cdc25C and (C) p-Cdc25C (Ser216). Protein levels were normalized to β-actin. The relative level of protein in the control group was set at 100%. The cells were treated with isorhamnetin (10, 50 or 100 µmol/l) for 24 h. *P<0.05 and **P<0.01 vs. the control group. Values are expressed as the mean ± standard deviation of triplicate results. p-Cdc, phosphorylated cell division cycle.
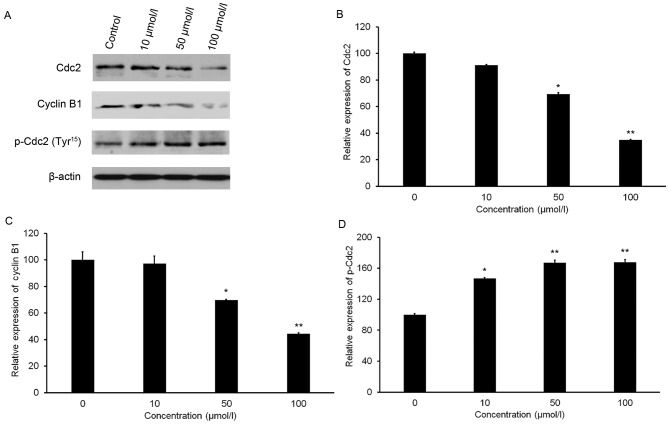
Isorhamnetin affects the protein levels of Cdc2, p-Cdc2 and cyclin B1
A slight increase in the protein level of p-Cdc2 (Tyr15) was observed after isorhamnetin treatment (Fig. 5A and B). Furthermore, isorhamnetin treatment resulted in a significant decrease in Cdc2 and cyclin B1 expression in a dose-dependent manner (Fig. 5A). Treatment with 100 µmol/l isorhamnetin resulted in 65.28 and 55.81% decreases in Cdc2 and cyclin B1 expression, respectively, compared with that in the control group (Fig. 5C and D).
Figure 5.
Effect of isorhamnetin on the levels of Cdc2, cyclin B1 and p-Cdc2 (Tyr15). (A) Protein levels were evaluated by western blot analysis. (B-D) Quantification of western blot results for (B) Cdc2, (C) cyclin B1 and (D) p-Cdc2. Protein expression was normalized against β-actin. The relative expression of protein in the control group was set at 100%. The cells were treated with isorhamnetin (10, 50 or 100 µmol/l) for 24 h. *P<0.05 and **P<0.01 vs. the control group. Values are expressed as the mean ± standard deviation of triplicate results. p-Cdc, phosphorylated cell division cycle.
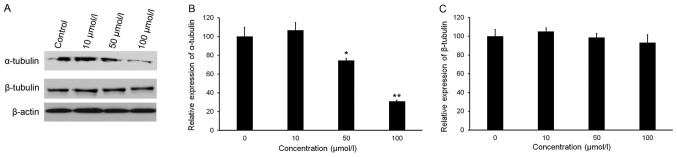
Isorhamnetin reduces the protein levels of tubulins in HeLa cells
Maintenance of a critical threshold of tubulin protein is essential for the function of microtubule networks. Analysis of tubulin protein levels indicated that isorhamnetin downregulated the expression of α-tubulin in a dose-dependent manner, but did not affect the expression of β-tubulin (Fig. 6A-C). The relative expression of α-tubulin in HeLa cells treated with 50 and 100 µmol/l isorhamnetin decreased by 25.6 and 69.0%, respectively, compared with that in the control group (Fig. 6C).
Figure 6.
Effect of isorhamnetin on the expression of α-tubulin and β-tubulin. The cells were treated with isorhamnetin (10, 50 or 100 µmol/l) for 24 h. (A) Protein expression was evaluated by western blot analysis. Quantification of western blot results for (B) α-tubulin and (C) β-tubulin. Protein levels were normalized to β-actin. The relative level of protein in the control group was set at 100%. *P<0.05 and **P<0.01 vs. the control group. Values are expressed as the mean ± standard deviation of triplicate results.
Discussion
The occurrence and progression of cervical carcinoma is associated with HPV infection as well as a wide range of cellular, epigenetic, genetic, immunological and environmental factors (19). The present study was the first to report the anti-proliferative function of isorhamnetin in HeLa cervical cancer cells.
Initially, the anti-proliferative effect of isorhamnetin on HeLa cells was assessed by the mitochondrial respiration-dependent MTT reduction method. However, as isorhamnetin has light absorption properties, it interfered with the result of the MTT assay. Therefore, the trypan blue exclusion method was used. The results indicated that isorhamnetin inhibited the proliferation of HeLa cells in a dose-dependent manner at 24, 48 and 72 h. Of note, the IC50 of isorhamnetin after 48 h of treatment was nearly three times greater than that after 24 h of treatment. As an underlying cause, it is possible that a repair system was activated in cells treated with isorhamnetin for 48 h, which will be investigated in further experiments.
The cell cycle determines cell proliferation and regulates complex processes that determine cell growth and division. All signaling pathways affecting the cell cycle must be precisely regulated in order to determine the fate of the cell. In almost all cancer cell types, a number of different mechanisms influence the normal cell cycle (20). To date, numerous anticancer drugs have been demonstrated to arrest the cell cycle at a certain phase (18,21). In the present study, the results indicated that isorhamnetin caused cell cycle arrest of HeLa cells at G2/M phase in a dose-dependent manner. Therefore, isorhamnetin may have potential as a treatment to prevent cancer growth.
Ataxia telangiectasia mutated (ATM) and ataxia telangiectasia and Rad3-related (ATR) proteins are kinases that are activated in the presence of cell damage signals, and induce either cell cycle arrest or apoptosis. Certain antineoplastic drugs have been demonstrated to activate these two checkpoint proteins (22,23). Activated ATM and ATR phosphorylate and activate two further checkpoint effector kinases, Chk1 and Chk2 (24), which phosphorylate a series of functionally associated cellular substrates (25). Chk1 is thought to be activated through phosphorylation of its Ser345 residue by ATR, and Chk2 is activated through phosphorylation of Thr68 by ATM (26,27). In the present study, treatment with isorhamnetin markedly increased p-Chk2 (Thr68), with no significant change in Chk2 total protein expression. However, no obvious alteration was observed in the protein level of p-Chk1 (Ser345). The results implied that isorhamnetin may cause cell cycle arrest at the G2/M phase via activation of the ATM-Chk2 pathway.
Activation of Chk1 or Chk2 leads to the phosphorylation of a variety of cell cycle regulatory proteins, including Cdc25C (28). Furthermore, p-Cdc25C (Ser216) provides a binding site for 14-3-3 family proteins, resulting in inhibition of Cdc25C-mediated G2/M transition (29). In the present study, western blot analysis demonstrated that Cdc25C protein expression was decreased, while its inactive form, p-Cdc25C (Ser216), was slightly elevated, indicating the inactivation of Cdc25C.
Cdc2 [also known as cyclin D kinase 1] and cyclin B1 constitute a complex called mitosis-promoting factor. This complex is involved in regulating G2/M transition (30). As cells enter into S and G2 phases, complexes of Cdc2 with B-type cyclins are in an inactive state due to Tyr15 phosphorylation of Cdc2. However, at the onset of the M phase, Cdc25 tyrosine phosphatases dephosphorylate Cdc2 at Tyr15. The Cdc2-cyclin B complexes trigger numerous events associated with the M phase (31,32). In order to elucidate the mechanism of cell cycle arrest upon isorhamnetin treatment, the protein levels of p-Cdc2 (Tyr15), Cdc2 and cyclin B1 were compared. Treatment with isorhamnetin dose-dependently decreased Cdc2 and cyclin B1, with a slight increase in p-Cdc2 (Tyr15). These changes may be associated with cell cycle arrest of HeLa cells in G2/M phase.
Microtubules are a component of the cytoskeleton. A large and diverse group of anticancer drugs derived from natural products target microtubules in cancer cells. Microtubules are composed of tubulin proteins (α-tubulin and β-tubulin), and are assembled dynamically through polymerization and de-polymerization. The normal regulation of microtubule assembly serves a key function in cells during M phase (33). Thus, in the present study, α-tubulin and β-tubulin expression were determined after treatment with isorhamnetin. Western blot analysis demonstrated that the expression of α-tubulin was reduced in isorhamnetin-treated cells, but there were no significant differences in β-tubulin expression. In summary, these results indicated that isorhamnetin disrupted tubulin polymerization, causing cell cycle arrest at G2/M phase.
In conclusion, the present study demonstrated that isorhamnetin significantly inhibits the proliferation of HeLa cells in vitro. The mechanism of the anti-proliferative effect of isorhamnetin was closely associated with cell cycle arrest at the G2/M phase through activation of the ATM-Chk2 pathway and disruption of microtubule function. The present study provides a basis for the development of isorhamnetin as a potential therapeutic drug for cervical cancer. Regarding the reproducibility of the results, it is required to test the effects of isorhamnetin on further cervical cancer cell lines e.g., SiHa and CaSki, in the future.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Projects of International Cooperation and Exchanges of the Ministry of Science and Technology in China (grant no. 2014DFR31230) and the National Natural Science Foundation of China (grant no. 31360382).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JW conceived and designed the study, performed the majority of the experiments and wrote the paper. HS performed the flow cytometric analysis. YB helped to design the study, reviewed and edited the manuscript and approved the final version to be published. JL performed the cell culture. LF helped to analyze the data. WS helped to analyze the data and was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Arbyn M, Castellsagué X, de Sanjosé S, Bruni L, Saraiya M, Bray F. Worldwide burden of cervical cancer in 2008. Ann Oncol. 2011;22:2675–2686. doi: 10.1093/annonc/mdr015. [DOI] [PubMed] [Google Scholar]
- 2.Farrand L, Oh SW, Song YS, Tsang BK. Phytochemicals: A multitargeted approach to gynecologic cancer therapy. Biomed Res Int. 2014;2014:890141. doi: 10.1155/2014/890141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 4.Kumar D, Basu S, Parija L, Rout D, Manna S, Dandapat J, Debata PR. Curcumin and ellagic acid synergistically induce ROS generation, DNA damage, p53 accumulation and apoptosis in HeLa cervical carcinoma cells. Biomed Pharmacother. 2016;81:31–37. doi: 10.1016/j.biopha.2016.03.037. [DOI] [PubMed] [Google Scholar]
- 5.Debata PR, Castellanos MR, Fata JE, Baggett S, Rajupet S, Szerszen A, Begum S, Mata A, Murty VV, Opitz LM, Banerjee P. A novel curcumin-based vaginal cream Vacurin selectively eliminates apposed human cervical cancer cells. Gynecol Oncol. 2013;129:145–153. doi: 10.1016/j.ygyno.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Thacker PC, Karunagaran D. Curcumin and emodin down-regulate TGF-β signaling pathway in human cervical cancer cells. PLoS One. 2015;10:e0120045. doi: 10.1371/journal.pone.0120045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maher DM, Bell MC, O'Donnell EA, Gupta BK, Jaggi M, Chauhan SC. Curcumin suppresses human papillomavirus oncoproteins, restores p53, Rb, and PTPN13 proteins and inhibits benzo[a]pyrene-induced upregulation of HPV E7. Mol Carcinog. 2011;50:47–57. doi: 10.1002/mc.20695. [DOI] [PubMed] [Google Scholar]
- 8.Narayanan BA, Geoffroy O, Willingham MC, Re GG, Nixon DW. p53/p21(WAF1/CIP1) expression and its possible role in G1 arrest and apoptosis in ellagic acid treated cancer cells. Cancer Lett. 1999;136:215–221. doi: 10.1016/S0304-3835(98)00323-1. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Chi G, Shen B, Tian Y, Feng H. Isorhamnetin ameliorates LPS-induced inflammatory response through downregulation of NF-κB signaling. Inflammation. 2016;39:1291–1301. doi: 10.1007/s10753-016-0361-z. [DOI] [PubMed] [Google Scholar]
- 10.Jiang JS, Shih CM, Wang SH, Chen TT, Lin CN, Ko WC. Mechanisms of suppression of nitric oxide production by 3-O-methylquercetin in RAW 264.7 cells. J Ethnopharmacol. 2006;103:281–287. doi: 10.1016/j.jep.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Yang JH, Kim SC, Shin BY, Jin SH, Jo MJ, Jegal KH, Kim YW, Lee JR, Ku SK, Cho IJ, Ki SH. O-Methylated flavonol isorhamnetin prevents acute inflammation through blocking of NF-κB activation. Food Chem Toxicol. 2013;59:362–372. doi: 10.1016/j.fct.2013.05.049. [DOI] [PubMed] [Google Scholar]
- 12.Saud SM, Young MR, Jones-Hall YL, Ileva L, Evbuomwan MO, Wise J, Colburn NH, Kim YS, Bobe G. Chemopreventive activity of plant flavonoid isorhamnetin in colorectal cancer is mediated by oncogenic Src and β-catenin. Cancer Res. 2013;73:5473–5484. doi: 10.1158/0008-5472.CAN-13-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang JH, Shin BY, Han JY, Kim MG, Wi JE, Kim YW, Cho IJ, Kim SC, Shin SM, Ki SH. Isorhamnetin protects against oxidative stress by activating Nrf2 and inducing the expression of its target genes. Toxicol Appl Pharmacol. 2014;274:293–301. doi: 10.1016/j.taap.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Li Q, Ren FQ, Yang CL, Zhou LM, Liu YY, Xiao J, Zhu L, Wang ZG. Anti-proliferation effects of isorhamnetin on lung cancer cells in vitro and in vivo. Asian Pac J Cancer Prev. 2015;16:3035–3042. doi: 10.7314/APJCP.2015.16.7.3035. [DOI] [PubMed] [Google Scholar]
- 15.Sobral F, Calhelha RC, Barros L, Dueñas M, Tomás A, Santos-Buelga C, Vilas-Boas M, Ferreira IC. Flavonoid composition and antitumor activity of bee bread collected in northeast Portugal. Molecules. 2017;22(pii):E248. doi: 10.3390/molecules22020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi X, Liu D, Zhang J, Hu P, Shen W, Fan B, Ma Q, Wang X. Extraction and purification of total flavonoids from pine needles of Cedrus deodara contribute to anti-tumor in vitro. BMC Complement Altern Med. 2016;16:245. doi: 10.1186/s12906-016-1249-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strober W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol. 2015;111:A3.B.1–3. doi: 10.1002/0471142735.ima03bs111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lv TZ, Wang GS. Antiproliferation potential of withaferin A on human osteosarcoma cells via the inhibition of G2/M checkpoint proteins. Exp Ther Med. 2015;10:323–329. doi: 10.3892/etm.2015.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. 2003;16:1–17. doi: 10.1128/CMR.16.1.1-17.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miao R, Wei J, Lv M, Cai Y, Du Y, Hui X, Wang Q. Conjugation of substituted ferrocenyl to thiadiazine as apoptosis-inducing agents targeting the Bax/Bcl-2 pathway. Eur J Med Chem. 2011;46:5000–5009. doi: 10.1016/j.ejmech.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Xu Y, Ching YP, Zhou Y, Chiu JF, Chen F, He QY. Multiple pathways were involved in tubeimoside-1-induced cytotoxicity of HeLa cells. J Proteomics. 2011;75:491–501. doi: 10.1016/j.jprot.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Meyn MS. Ataxia-telangiectasia and cellular responses to DNA damage. Cancer Res. 1995;55:5991–6001. [PubMed] [Google Scholar]
- 23.Shiloh Y. Ataxia-telangiectasia: Closer to unraveling the mystery. Eur J Hum Genet. 1995;3:116–138. doi: 10.1159/000472285. [DOI] [PubMed] [Google Scholar]
- 24.Khanna KK, Jackson SP. DNA double-strand breaks: Signaling, repair and the cancer connection. Nat Genet. 2001;27:247–254. doi: 10.1038/85798. [DOI] [PubMed] [Google Scholar]
- 25.O'Neill T, Giarratani L, Chen P, Iyer L, Lee CH, Bobiak M, Kanai F, Zhou BB, Chung JH, Rathbun GA. Determination of substrate motifs for human Chk1 and hCds1/Chk2 by the oriented peptide library approach. J Biol Chem. 2002;277:16102–16115. doi: 10.1074/jbc.M111705200. [DOI] [PubMed] [Google Scholar]
- 26.Zhao H, Piwnica-Worms H. ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol Cell Biol. 2001;21:4129–4139. doi: 10.1128/MCB.21.13.4129-4139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahn JY, Schwarz JK, Piwnica-Worms H, Canman CE. Threonine 68 phosphorylation by ataxia telangiectasia mutated is required for efficient activation of Chk2 in response to ionizing radiation. Cancer Res. 2000;60:5934–5936. [PubMed] [Google Scholar]
- 28.Hirao A, Cheung A, Duncan G, Girard PM, Elia AJ, Wakeham A, Okada H, Sarkissian T, Wong JA, Sakai T, et al. Chk2 is a tumor suppressor that regulates apoptosis in both an ataxia telangiectasia mutated (ATM)-dependent and an ATM-independent manner. Mol Cell Biol. 2002;22:6521–6532. doi: 10.1128/MCB.22.18.6521-6532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agarwal C, Tyagi A, Agarwal R. Gallic acid causes inactivating phosphorylation of cdc25A/cdc25C-cdc2 via ATM-Chk2 activation, leading to cell cycle arrest, and induces apoptosis in human prostate carcinoma DU145 cells. Mol Cancer Ther. 2006;5:3294–3302. doi: 10.1158/1535-7163.MCT-06-0483. [DOI] [PubMed] [Google Scholar]
- 30.Pines J, Hunter T. Human cyclin A is adenovirus E1A-associated protein p60 and behaves differently from cyclin B. Nature. 1990;346:760–763. doi: 10.1038/346760a0. [DOI] [PubMed] [Google Scholar]
- 31.Russell P, Nurse P. The mitotic inducer nim1+ functions in a regulatory network of protein kinase homologs controlling the initiation of mitosis. Cell. 1987;49:569–576. doi: 10.1016/0092-8674(87)90459-4. [DOI] [PubMed] [Google Scholar]
- 32.Kumagai A, Dunphy WG. The cdc25 protein controls tyrosine dephosphorylation of the cdc2 protein in a cell-free system. Cell. 1991;64:903–914. doi: 10.1016/0092-8674(91)90315-P. [DOI] [PubMed] [Google Scholar]
- 33.Jordan MA. Mechanism of action of antitumor drugs that interact with microtubules and tubulin. Curr Med Chem Anticancer Agents. 2002;2:1–17. doi: 10.2174/1568011023354290. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.