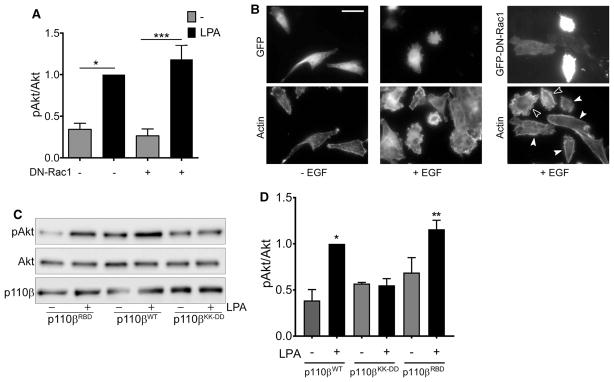
Figure 3. LPA-stimulated activation of PI3Kβ does not require Rac1 binding.
(A) Parental MDA-MB-231 cells were transfected with GFP or GFP-DN-Rac1 and stimulated with or without 10 μM LPA for 5 min. pAkt/Akt ratios in each experiment were normalized to that seen in LPA-stimulated cells without DN-Rac1. The data represent the mean ± SEM from three independent experiments. Western blot data from one of the three experiments is shown in Figure 1E. (B) Representative micrographs of parental MDA-MB-231 cells transfected with GFP or GFP-DN-Rac1 (upper panels) and stimulated without or with EGF. Cells were stained with rhodamine phalloidin to visualize F-actin (lower panels). Open arrowheads indicate untransfected cells that show membrane ruffiing. Closed arrowheads show transfected cells with reduced ruffiing. Scale bar = 50 μm. (C) Stable p110β knockdown cells were transiently transfected with wild-type p110β, p110βKK-DD, or p110βRBD. Forty-eight hours after transfection, cells were starved overnight and then stimulated with 10 μM LPA for 5 min. Cells were lysed and blotted for p110β, Akt, and pT308-Akt. (D) Quantitation of pAkt/Akt immunoblots in Figure 3D. pAkt/Akt ratios in each experiment were normalized to that seen in cells expressing wild-type p110β and stimulated with LPA. The data represent the mean ± SEM from three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001; ns: not significant.