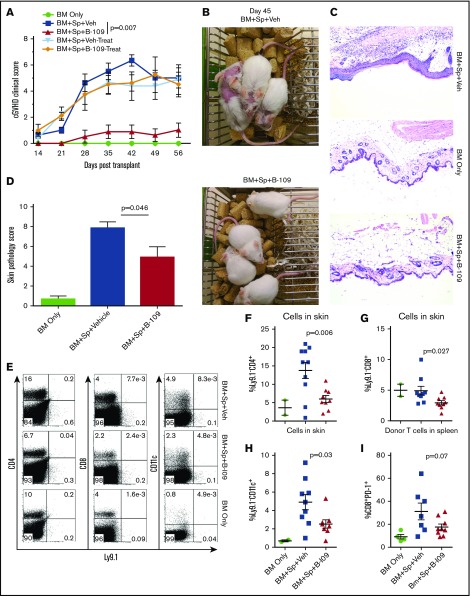
Figure 6.
Pharmacologic inhibition of IRE-1α/XBP-1 prevents cGVHD in MHC-matched BMT model. Lethally irradiated BALB/c recipients were transplanted with TCD-BM (5 × 106/mouse) from B10.D2 donors with (n = 30) or without (n = 4) whole splenocytes at 5 × 106/mouse. Groups were either given no treatment (n = 4), given daily IP injection of DMSO vehicle alone starting on day 0 and continued for 3 weeks (days 0-21; n = 10) or beginning on day 21 (days 21-42; n = 5), or IP injected with B-I09 at a dose of 25 mg/kg beginning at day 0 and continued for 3 weeks (days 0-21; n = 10), or beginning on day 21 (days 21-42; n = 5). Recipient mice were monitored for cGVHD clinical scores (A) until experiment endpoint on day 60. On day 45, images were taken of prophylactically treated vehicle and B-I09 groups (B). Skin biopsies were sectioned and stained with hematoxylin and eosin (magnification ×200) (C) and analyzed by an independent pathologist for signs of cGVHD skin damage (D). On day 60, mice were killed, and spleens and trunk skin were excised for processing into single-cell suspension for flow cytometric analysis of donor CD229.1− (Ly9.1−) CD4, CD8, and CD11c lymphocyte skin infiltrates (E). Quantification of CD4 (F), CD8 (G), and CD11c (H) cells in skin is shown. Splenic donor CD8+ T cells were analyzed via flow cytometry for expression of PD-1 (I). Data shown in panels A-C are representative of 2 separate experiments. Data shown in panel D are pooled from 2 replicate experiments. P < .05 indicates statistical significance. Data in panel E are representative flow plots from 2 separate experiments, which are quantified as pooled data in panels F-I. Statistical analysis of cGVHD clinical scores was performed using a Mann-Whitney U test of the entire experimental time course. P < .05 indicates statistical significance.